��Ŀ����
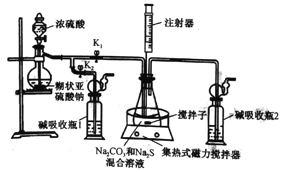
����Ŀ�������������ܶ࣬����H2SO4��H2SO3��SO2��Na2SO3��BaSO4��CuSO4��Na2SO4��7�ֳ����ĺ�����ش��������⣺
(1)H2SO3ת��Ϊ�����������γɵ���Ҫ����֮һ��д���䷴Ӧ�Ļ�ѧ����ʽ������������ת�Ƶķ������Ŀ��_______________________________________��
(2)�����£�����������Ũ�����У����������α��Ͻ���Ϊ�����˶ۻ�����������Ϊδ������Ӧ��Ϊ��֤�˹��̣�ijͬѧ����˼�������������ʵ�飺����Ũ���ᴦ����������ϴ��������CuSO4��Һ�У�����������_______________________�������˶ۻ�������������____________________����δ������Ӧ��
(3)��Na2SO3���շ�������SO2��Ⱦ��һ�ַ�������ԭ��Ϊ(�û�ѧ����ʽ��ʾ)�� _______________________________________________________________��
(4)����SO2����Ⱦ�����Ϊ�����ҹ�����̽����һ����������CO��ԭSO2�õ�������ķ�������ȥSO2���÷�Ӧ�Ļ�ѧ����ʽ��______________________��
���𰸡�![]() ===2H2SO4��ɫ���������к�ɫ��������Na2SO3��SO2��H2O===2NaHSO3SO2��2CO
===2H2SO4��ɫ���������к�ɫ��������Na2SO3��SO2��H2O===2NaHSO3SO2��2CO![]() S��2CO2
S��2CO2
��������
(1)�����ᱻ�����������ᣬ���ݵ���ת���غ���ƽ��������ӵ���ת����Ŀ�뷽���磺 ![]() =2H2SO4 �� (2)�ۻ����ڽ�����������һ�����ܵ������ﱣ��Ĥ����ֹ�ڲ����з�Ӧ���������ۻ���������Ĥ�����������ܺ�����ͭ�����û���Ӧ������������ɫ���������������ۻ������������к�ɫ����������(3) �������ƺͶ�������Ӧ�������������ƣ���Ӧ����ʽΪ��Na2SO3��SO2��H2O=2NaHSO3 (4) ��CO��ԭSO2�õ�������ķ�������ȥ��һ����̼���ɶ�����̼����Ӧ����ʽΪ��SO2��2CO
=2H2SO4 �� (2)�ۻ����ڽ�����������һ�����ܵ������ﱣ��Ĥ����ֹ�ڲ����з�Ӧ���������ۻ���������Ĥ�����������ܺ�����ͭ�����û���Ӧ������������ɫ���������������ۻ������������к�ɫ����������(3) �������ƺͶ�������Ӧ�������������ƣ���Ӧ����ʽΪ��Na2SO3��SO2��H2O=2NaHSO3 (4) ��CO��ԭSO2�õ�������ķ�������ȥ��һ����̼���ɶ�����̼����Ӧ����ʽΪ��SO2��2CO![]() S��2CO2��
S��2CO2��

 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�����Ŀ�����г��ӷ���ѡ�ô������
����(������Ϊ����) | ���ӷ��� | |
A | ������(��) | ���� |
B | ��ϩ(SO2) | NaOH��Һ��ϴ�� |
C | ����(��ϩ) | ��ˮ����Һ |
D | ������Һ(NaCl) | ���� |
A.AB.BC.CD.D