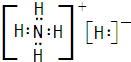��Ŀ����
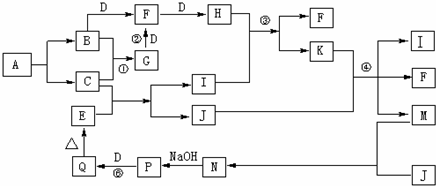
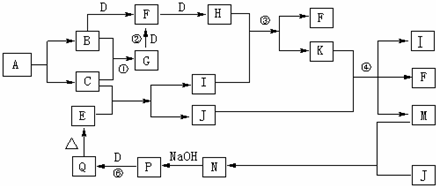
���ӻ�����Aֻ������Ԫ����ɣ�����Ԫ�ص�������Ϊ14��5�����������ӵ�ɱ�Ϊ1��A���ȶ���һ�������±�ը����B��C������Ϊ������ʵ�ת����ϵ������B��C��D��JΪ���ʣ�����ֻ��JΪ���壬EΪ����ɫ��������壬��Ӧ�١��ڶ��ǹ�ҵ�����ϵ���Ҫ��Ӧ���������������ԣ��ܷ�Ӧ��K������

�ش��������⣺
��1��A�ĵ���ʽΪ______��
��2��K��Ũ��Һ���û��ɫ����ɫ�����з�����Ӧ�Ļ�ѧ����ʽΪ______��
��3��ʵ���Ҽ��麬A�е������ӵĿ�������ʱ�IJ���������______��
��4����Ӧ�ڢĻ�ѧ����ʽΪ����______����______��

�ش��������⣺
��1��A�ĵ���ʽΪ______��
��2��K��Ũ��Һ���û��ɫ����ɫ�����з�����Ӧ�Ļ�ѧ����ʽΪ______��
��3��ʵ���Ҽ��麬A�е������ӵĿ�������ʱ�IJ���������______��
��4����Ӧ�ڢĻ�ѧ����ʽΪ����______����______��
�⣨1�����ӻ�����Aֻ������Ԫ����ɣ�����Ԫ�ص�������Ϊ14��5�����������ӵ�ɱ�Ϊ1��A���ȶ���һ�������±�ը����B��C����B��CΪ���嵥�ʣ��������ڱ�����������Ԫ�صĵ���Ϊ�������H��N��O��F��Cl����Ԫ�أ����γɵij���������Ϊ��NH4+���������Ԫ�ص�������Ϊ14��5�����������ӵ�ɱ�Ϊ1����ȷ��AΪNH4H������ʽΪ��
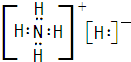
�ʴ�Ϊ��
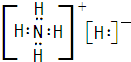
��
��2�����ݵ�Ԫ�صĵ��ʺͻ���������ʷ���BΪN2��CΪH2��DΪO2��FΪNO��HΪNO2��GΪNH3����Ӧ��ΪNO2��ˮ��Ӧ���������NO��Ũ������Һ���û��ɫ����Ϊ�ֽ����ɵ�NO2�ܽ���������Һ�У�
��Ӧ�Ļ�ѧ����ʽΪ��4HNO3��Ũ��=2H2O+4NO2��+O2����
�ʴ�Ϊ��4HNO3��Ũ��=2H2O+4NO2��+O2����
��3��ʵ���Ҽ��麬A�е������ӵĿ�������ʱ�IJ��������ǣ���ǿ����Һ���ȣ��ò���ճʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲���ֽ�Ƿ����ɫ��
�ʴ�Ϊ����ǿ����Һ���ȣ��ò���ճʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲���ֽ�Ƿ����ɫ��
��4����Ӧ���ǰ����Ĵ�������Ӧ����ѧ����ʽΪ��4NH3+5O2
4NO+6H2O����Ӧ���������������������е���������Ϊ���������ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
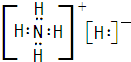
�ʴ�Ϊ��
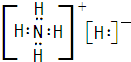
��
��2�����ݵ�Ԫ�صĵ��ʺͻ���������ʷ���BΪN2��CΪH2��DΪO2��FΪNO��HΪNO2��GΪNH3����Ӧ��ΪNO2��ˮ��Ӧ���������NO��Ũ������Һ���û��ɫ����Ϊ�ֽ����ɵ�NO2�ܽ���������Һ�У�
��Ӧ�Ļ�ѧ����ʽΪ��4HNO3��Ũ��=2H2O+4NO2��+O2����
�ʴ�Ϊ��4HNO3��Ũ��=2H2O+4NO2��+O2����
��3��ʵ���Ҽ��麬A�е������ӵĿ�������ʱ�IJ��������ǣ���ǿ����Һ���ȣ��ò���ճʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲���ֽ�Ƿ����ɫ��
�ʴ�Ϊ����ǿ����Һ���ȣ��ò���ճʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲���ֽ�Ƿ����ɫ��
��4����Ӧ���ǰ����Ĵ�������Ӧ����ѧ����ʽΪ��4NH3+5O2
| ||
�ʴ�Ϊ��4NH3+5O2
| ||

��ϰ��ϵ�д�
�����Ŀ
����˵���в���ȷ���ǣ� ��
| A����XY3������Xԭ�Ӵ����������ε����ģ���XY3����Ϊ�Ǽ��Է��� |
| B��C2H5OH��C2H5Br��ȣ�ǰ�ߵķе�Զ���ں��ߣ���ԭ����ǰ�ߵķ��Ӽ������� |
| C��ͬ���ڢ�A��Ԫ�غ� ��A��Ԫ��֮��ֻ���γ����ӻ����� |
| D�������ַǽ���Ԫ����ɵĻ����������ֻ�����м��Լ��������зǼ��Լ� |