��Ŀ����
����Ŀ���ϳɰ��������ѧ������չʷ�ϵ�һ���ش�ͻ�ƣ��о�����Һ����һ�����õĴ������ʡ�
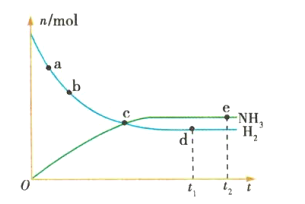
��1�� �����ֽⷴӦ���Ȼ�ѧ����ʽ���£�2NH3��g�� ��N2��g����3H2��g������H������N��N����H��H����N��H���ļ��ֱܷ����a��b��c����λ��kJ��mol1������������Ӧ����H��________kJ��mol1��
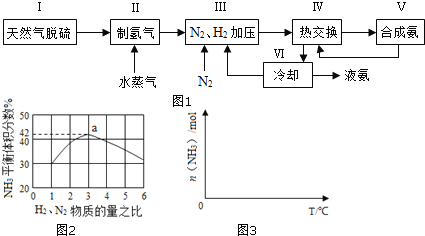
��2�� �о��������������ɼ��ٰ����ķֽ⡣�±�Ϊij�¶��µ������IJ�ͬ�����ֱ����Ũ�Ȱ����ֽ����������ij�ʼ���ʣ�mmol��min1����
���� | Ru | Rh | Ni | Pt | Pd | Fe |
��ʼ���� | 7.9 | 4.0 | 3.0 | 2.2 | 1.8 | 0.5 |
��ͬ���������£������ֽⷴӦ���������________����д�����Ļ�ѧʽ����
��3�� �绯ѧ��Ҳ�ɺϳɰ�����ͼ���õ��¹������ӵ�����Ϊ����ʣ���PtC3N4�������������H2��g����N2��g���ϳ�NH3��ԭ��ʾ��ͼ��
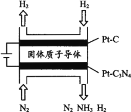
PtC3N4�缫��Ӧ����NH3�ĵ缫��Ӧʽ________��
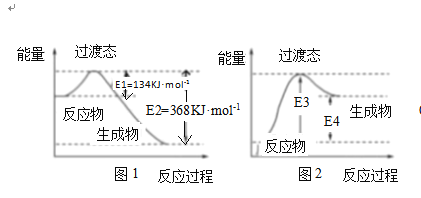
��4������̬������Ϊ����ѧ��Ӧ������ͨ������ײ������ɵģ����Ǵӷ�Ӧ�ﵽ������Ĺ����о���һ���������Ĺ���̬����ͼ1Ϊ1molNO2��1molCOǡ�÷�Ӧ����CO2��NO�����е������仯ʾ��ͼ
����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��______
�����ܱ������н��е�������Ӧ�ǿ��淴Ӧ��ͼ2��ijѧ��ģ��ͼ1������NO+CO2 =NO2+CO�������仯ʾ��ͼ����ͼ��E3=______kJmol-1
���𰸡�6c��a��3b Fe N2��6e��6H+=2NH3 NO2��g��+CO��g���TNO��g��+CO2��g����H=-234kJmol-1 368
��������
(1)��Ӧ����H����Ӧ��ļ��ܺ�-������ļ��ܺͣ�
(2)�����ɽ��ͷ�Ӧ��ܣ��ӿ췴Ӧ���ʣ���Ч��Խ�ߣ���Ӧ����Խ�죬��Ӧ���Խ�ͣ�
(3)�õ��¹������ӵ�����Ϊ����ʣ���Pt-C3N4�������������H2(g)��N2(g)�ϳ�NH3�����������ӵõ����������������͵��������������ɰ�����
(4)�ٸ÷�Ӧ���ʱ�=(134-368)kJ/mol=-234kJ/mol��
�ڸ���ͼ֪���÷�Ӧ��ͼ1�е��淴Ӧ��E3Ϊͼ1�е�E2��
(1)��Ӧ2NH3(g) ��N2(g)��3H2(g)����H����Ӧ��ļ��ܺ�-������ļ��ܺ�=(ckJ��mol1)��6-(akJ��mol1)��1-(bkJ��mol1)��3= (6c��a��3b)kJ��mol1��
(2)�����ɽ��ͷ�Ӧ��ܣ��ӿ췴Ӧ���ʣ���Ч��Խ�ߣ���Ӧ����Խ�죬��Ӧ���Խ�ͣ��ɱ������ݿ�֪��ʹ��Fe������ʱ����Ӧ������ͣ�˵�������ֽⷴӦ������
(3)�绯ѧ��Ҳ�ɺϳɰ����õ��¹������ӵ�����Ϊ����ʣ���Pt-C3N4�������������H2(g)��N2(g)�ϳ�NH3�Ĺ����У����������ӵõ����������������͵��������������ɰ������缫��ӦʽΪN2��6e��6H+=2NH3��
(4)�ٸ÷�Ӧ���ʱ�=(134-368)kJ/mol=-234kJ/mol���÷�Ӧ�Ȼ�ѧ����ʽΪNO2(g)+CO(g)�TNO(g)+CO2(g)��H=-234kJmol-1��
�ڸ���ͼ֪���÷�Ӧ��ͼ1�е��淴Ӧ��E3Ϊͼ1�е�E2Ϊ368kJ/mol��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�����Ŀ��NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ����ҵ�ϳ�����������Ҫ�ɷ�ΪAl2O3��������SiO2��Fe2O3���ʣ��������������NH4Al(SO4)212H2O���乤������ͼ���£�
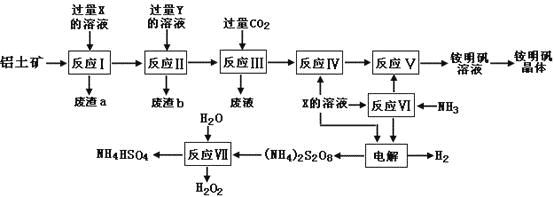
��1������a��b �ijɷֱַ��ǣ�_________��_____________����д���ƣ�
��2������ͼ��X�Ļ�ѧʽΪ��_______________��
��3����Ӧ������ӷ���ʽΪ��_________________________________________�����������Һ�л�����������IJ�������Ϊ����������ƣ�_________����ȴ�ᾧ������ϴ�ӡ�
��4���������[(NH4)2S2O8]�ڹ�ҵ�������й㷺����;��������Ϊ��������Ư�����㷺���������ع�ҵ���������ۺϵ�����������ά��ҵ���ѽ������������Ĺ����������ö��Ե缫���X�뷴Ӧ���������ʵĻ����Һ���Եõ�������李�
д��������Ӧʽ��______________________________________________��
��5����Ӧ���Ļ�ѧ����ʽΪ��________________________________________��
NH4HSO4��Һ������Ũ���ɴ�С˳��Ϊ��_____________________________��
��6�������������Һ����μ�������������Һ�������ܷ����ķ�Ӧ��______����ѡ����ĸ��
A��4NH4Al(SO4)2+3Ba(OH)2��2(NH4)2SO4+3BaSO4��+ Al2(SO4)3+2Al(OH)3�� |
B��2NH4Al(SO4)2+4Ba(OH)2��(NH4)2SO4+3BaSO4��+Ba(AlO2)2 |
C��2NH4Al(SO4)2+3Ba(OH)2��(NH4)2SO4+3BaSO4��+2Al(OH)3�� |
D��NH4Al(SO4)2+2Ba(OH)2��NH3��H2O+2BaSO4��+ Al(OH)3�� |