��Ŀ����
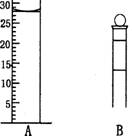
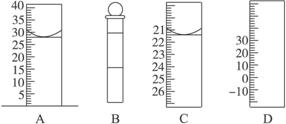
��.��ͼΪ���������IJ��ֽṹ(�е��������Ŵ�)
ͼA��Һ����ʾ��Һ�����Ϊ mL�����������������е�ij�ֲ���һҺ��������ƽ��ʱ����Ϊn mL������ʱ����Ϊm mL����m��n������ʹ�õ������� (����ĸ���)��
��.ijѧ���ñ�0.25 mol��L��1 NaOH��Һ�ζ������ʵ��������£�
A������ʽ�ζ���ȡϡH2SO4 25.00 mL��ע����ƿ�У�����ָʾ����
B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ� C��������ˮϴ�ɾ��ζ��ܡ�
D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0������2��3 cm�����ٰѼ�ʽ�ζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����¡�
E�����ζ����Ƿ�©ˮ�� F����ȡ��ƿ�����ظ�����һ�Ρ�
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȡ�
�ٵζ���������ȷ˳����(�������)�� ��������������������������������
�ڸõζ�������Ӧѡ�õ�ָʾ���ǣ� �������� ��
����G���������ȷ����? ��
�ܼ�ʽ�ζ���������ˮ��ϴ��δ�ñ�Һ��ϴ���µζ����(�ƫС������ƫ��ǡ�ú��ʡ�) ��ԭ���� ��
�ݼ����������ȷ����Һ������22.18mL,�������������Һ�����ʵ���Ũ�ȣ���������ȷ��С������λ) mol��L��1
��.��֪����ʱ����ĵ��볣��Ϊ1.8��10��5��һˮ�ϰ��ĵ��볣��Ϊ1.8��10��5��������Mg(OH)2����Һ�У��μ���������NH4Cl��Һ�������ܽ⡣���ڹ�����ܽ⡣
��ͬѧ��Ϊ��������þ����Һ�д�������ƽ�⣺
Mg(OH)2 ![]() Mg2+ +2OH-
Mg2+ +2OH-
����NH4Cl��Һ������NH4+ˮ�����H+�к���Mg(OH)2���������OH-��ʹƽ�����Ƶ���Mg(OH)2 �ܽ⡣
����ͬѧȴ��ΪNH4Cl���������NH4+�����OH���������������NH3��H2O��ʹc(OH-)��С��ƽ�����ƶ�����Mg(OH)2 �ܽ⡣��Ʒ������ۼ�����λͬѧ�Ĺ۵㣺
_____________________________________ ______________________________
_________________________________________________________________________
�����б�ע���⣬����ÿ��1�֣���12�֣�
��. 28 C
��. ��ECDBAGF����ECBADGF����2�֣��ڷ�̪������Ҳ�ɣ��۵������һ��NaOH��Һ����ҺͻȻ��ɷۺ�ɫ������Ӳ���ɫ�����ü��ȡ�����ʱ�����ɫΪ��ɫ����ƫ�ζ����ڱ��ϵ�ˮĤ������Һϡ�ͣ�ʹ�������ƫ���0.11
��. ��������þ����Һ�У��������粒��壬�۲������������ܽ�����ͬѧ�۵���ȷ�������岻�ܽ⣬���ͬѧ�۵���ȷ����3�֣�

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
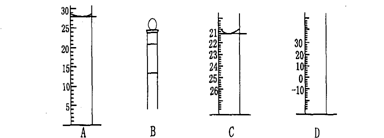
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д���.��ͼΪ���������IJ��ֽṹ(�е��������Ŵ�)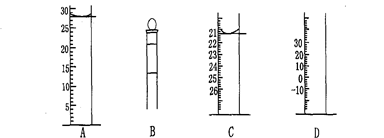
ͼA��Һ����ʾ��Һ�����Ϊ mL�����������������е�ij�ֲ���һҺ��������ƽ��ʱ����Ϊn mL������ʱ����Ϊm mL����m��n������ʹ�õ������� (����ĸ���)��
��.ijѧ���ñ�0.25 mol��L��1 NaOH��Һ�ζ������ʵ��������£�
| A������ʽ�ζ���ȡϡH2SO4 25.00 mL��ע����ƿ�У�����ָʾ���� | |
| B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ� | C��������ˮϴ�ɾ��ζ��ܡ� |
| D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0������2��3 cm�����ٰѼ�ʽ�ζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����¡� |
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȡ�
�ٵζ���������ȷ˳����(�������)�� ��������������������������������
�ڸõζ�������Ӧѡ�õ�ָʾ���ǣ� ����������
����G���������ȷ����? ��
�ܼ�ʽ�ζ���������ˮ��ϴ��δ�ñ�Һ��ϴ���µζ����(�ƫС������ƫ��ǡ�ú��ʡ�) ��ԭ���� ��
�ݼ����������ȷ����Һ������22.18mL,�������������Һ�����ʵ���Ũ�ȣ���������ȷ��С������λ) mol��L��1
��.��֪����ʱ����ĵ��볣��Ϊ1.8��10��5��һˮ�ϰ��ĵ��볣��Ϊ1.8��10��5��������Mg(OH)2����Һ�У��μ���������NH4Cl��Һ�������ܽ⡣���ڹ�����ܽ⡣
��ͬѧ��Ϊ��������þ����Һ�д�������ƽ�⣺
Mg(OH)2
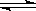 Mg2+ +2OH-
Mg2+ +2OH-����NH4Cl��Һ������NH4+ˮ�����H+�к���Mg(OH)2���������OH-��ʹƽ�����Ƶ���Mg(OH)2 �ܽ⡣
����ͬѧȴ��ΪNH4Cl���������NH4+�����OH���������������NH3��H2O��ʹc(OH-)��С��ƽ�����ƶ�����Mg(OH)2 �ܽ⡣��Ʒ������ۼ�����λͬѧ�Ĺ۵㣺
_____________________________________ ______________________________
_________________________________________________________________________
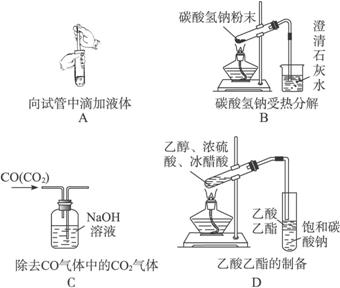


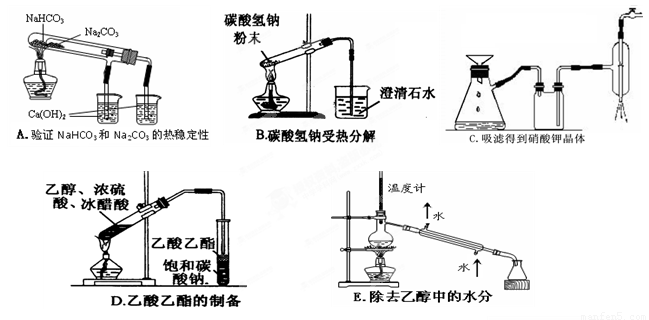 (2 ) ����ʵ��û�д������_______________________
(2 ) ����ʵ��û�д������_______________________