��Ŀ����
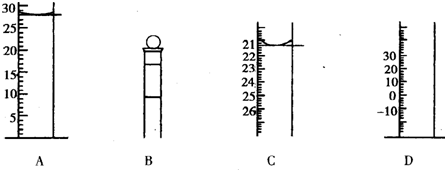
��1����ͼΪ���������IJ��ֽṹ(�е��������Ŵ�)Aͼ��Һ����ʾ��Һ�����Ϊ mL�����������������е�ij�ֲ���һҺ��������ƽ��ʱ����ΪN mL������ʱ����ΪM mL����M>N������ʹ�õ�������____ (����ĸ���)��
��2���ڻ�ѧ�����У�������KMnO4����Һ������KMnO4�����������²�̫�ȶ����������ֱ������ȷ���ʵ���Ũ�ȵ�KMnO4��Һ��ʵ����һ���ȳ�ȡһ��������KMnO4���壬����ɴ���Ũ�ȵ�KMnO4��Һ�����������ȶ�����Է��������ϴ�Ļ����ʲ�����[Mr(Na2C2O4)��134��0]�Դ����KMnO4��Һ���б궨����������Ƶ�KMnO4��Һ��ȷŨ�ȣ���Ӧԭ��Ϊ��5C2O42����2MnO4����16H����10CO2����2Mn2+��8H2O
�����DZ궨KMnO4��Һ��ʵ�鲽�裺
����һ���ȴ���Ũ��ԼΪ0��15mol��L-1�ĸ��������Һ500 mL��
�������ȷ��ȡNa2C2O4����m g������ƿ�У�������ˮ�ܽⲢ��ϡ�����ữ��������70��80�棬�ò���һ������������Һ���еζ�����¼������ݡ�
�������� ��
�����ģ�����ø�����ص����ʵ���Ũ�ȡ��Իش��������⣺
�ٸõζ�ʵ�� ������Ҫ������Ҫ������ָʾ����
�ڲ�����еζ�����ͼʾ��ȷ����__________�����ţ���
�۲�����ĵζ������¶ȱ仯�������ԣ������������з���ǰһ����Һ��ɫ�������м����ɫ���Ա�죬������ɫ�ֱ������Ը���Ӱ�컯ѧ��Ӧ���ʵ�������������Һ��ɫ���Ա���ԭ�������_______ ������ֱ�����ԭ���� ������д���������IJ������� ������m��ƽ����ֵΪ1.340g���ζ���KMnO4��Һƽ������Ϊ25.00mL����KMnO4��Һ��Ũ��Ϊ mol��L-1��
��1��28.0��C
��2���ٲ���Ҫ ��A
�����ɵ�Mn2+Ϊ������ʹ��Ӧ���ʱ��Ӧ��Ũ�ȼ�С���������ʱ�С
���ظ���������������Σ�
��0.1600��д0.16�� 0.160������ȷ��
����