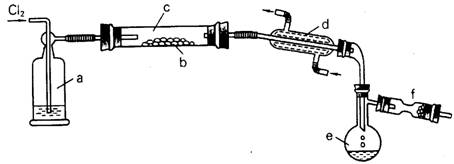
��Ŀ����
��֪��ˮ��Cl2������ǿ�����ԣ��������ӻ�ԭ������ǿ��˳��ΪI- >Fe2+ >Br-������200mL��FeI2��FeBr2��0.1mol�Ļ����Һ����������μ�����ˮ���ٶ�Cl2����ֻ�����ʷ�Ӧ��������������Ӧ������1������ˮ����0.1mol Cl2����ԭ����������Һ�к��е�������Ϊ______________��ʣ���Fe2+�����ʵ���Ϊ________��
��2����ԭ��Һ��Br-��һ�뱻������������Cl2������Ϊ________����������Һ���Ϊ400mL��������Ҫ����������________�������ʵ���Ũ��Ϊ ________��
��3�����ж�Cl2��I2��Fe3+��Br2����������������������ǿ������˳��Ϊ________________��
��4����ԭ�����Һ����������ȫ�����������ٵ���������ˮ����I2ȫ����Cl2������HIO3��ǿ�ᣩ����д���˷�Ӧ�����ӷ���ʽ��____________________��
��1��Cl-��Br-��0.2mol
��2��17.75g��Fe3+��0.5mol?L-1
��3��Cl2>Br2>Fe3+>I2
��4��I2+5Cl2+6H2O=2
 +10Cl-+12H+
+10Cl-+12H+�����������

±��Ԫ�صĵ��ʺͻ�����ܶ࣬���ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǡ�
��1��±��Ԫ��λ�����ڱ���_______������ļ۵����Ų�ʽΪ____________________��
��2���ڲ�̫ϡ����Һ�У���������Զ����ӵ�(HF)2��ʽ���ڵġ�ʹ�������ӵϵ���������________________��
��3��������±��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����_________��
| �� | �� | �� | �� | �� |
��һ������ ��kJ/mol�� | 1681 | 1251 | 1140 | 1008 | 900 |
��4����֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6�� ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4������������������������������
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4������������������������������
��5������ˮ�е��ܽ����ȻС�����ڵ⻯����Һ���ܽ��ȴ������������������Һ�з������з�ӦI-+I2=I3-����KI3���Ƶģ�����CsICl2������֪CsICl2���ȶ��������ֽ⣬���������ɾ����ܸ�������ʣ�����������_____ʽ������
A��CsICl2=CsCl+ICl??????? B��CsICl2=CsI+Cl2
��6����֪ClO2-Ϊ���ͣ�������ԭ����Χ���ĶԼ۲���ӡ�ClO2-������ԭ�ӵ��ӻ��������Ϊ___________��д��һ��ClO2-�ĵȵ�����__________��
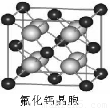
��7����֪CaF2���壨��ͼ�����ܶ�Ϊ�� g��cm-3��NAΪ�����ӵ�������������ڵ�����Ca2+�ĺ˼��Ϊa cm����CaF2����Է����������Ա�ʾΪ___________��
 HCl+HClO��CaCO3����ˮ�е�HCl��Ӧ��ʹc��H+����С��ƽ�������ƶ���HClOŨ������Ư������ǿ
HCl+HClO��CaCO3����ˮ�е�HCl��Ӧ��ʹc��H+����С��ƽ�������ƶ���HClOŨ������Ư������ǿ