��Ŀ����
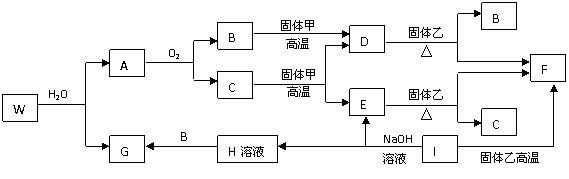
��ͼ��A��EΪ�����������ʣ�D��GΪ�������嵥�ʣ�B������Ϊ��ɫ��ζ��Һ�壬E��F�����������������ռ���Һ��Ӧ���ݴ˻ش��������⣺

(1)A�����ڱ��е�λ����_________��E��ԭ�ӽṹʾ��ͼ��_________��G��ͬ��������ķ���ʽ��_________��
(2)д���٢�������Ӧ�Ļ�ѧ����ʽ
��__________________________________����_________________________________��
(3)F���ʱ�����ĵ缫��ӦʽΪ__________________���ö��Ե缫���B��D��Gʱ��Ϊ�ӿ������ʣ��ֲ�Ӱ�����ɷ֣��������м������������е�(����ĸ)__________________��
A.CuCl2��Һ B.NaCl��Һ C.ϡH2SO4 D.NaOH��Һ

(2)��3Fe+4H2O![]() Fe3O4+4H2�� ��8Al+3Fe3O4
Fe3O4+4H2�� ��8Al+3Fe3O4![]() 9Fe+4Al2O3
9Fe+4Al2O3
(3)4 Al3++12e-====4Al��Al3++3e-=Al C��D

��13 �֣�
��1��CuSO4��Һ����ѧ��ѧ����ũҵ�����г�����һ���Լ���ijͬѧ����CuSO4��Һ����������ʵ��̽����
�� ͼһ�Ǹ��ݷ�ӦZn + CuSO4 =" Cu" + ZnSO4��Ƴɵ�пͭԭ��ء�����ʼ���Һ�� ���ZnSO4����CuSO4������Һ��Cu���ĵ缫��Ӧʽ�� ��
��ͼ���У����Ǽ���ȼ�ϵ�أ��������ҺΪKOH��Һ���Ľṹʾ��ͼ����ͬѧ���ڢ���ʵ�����϶�ͭ����b��ͨ����� ���CH4����O2������a���缫�Ϸ����ĵ缫��Ӧʽ�� ��
��2����������CuSO4��5H2O������ʯ�Һ�ˮ��һ��������ϣ����ɵõ�������Һ��ɱ������������Ч�ɷ�Ϊ���ܵļ�ʽ����ͭ[xCuSO4 ��yCu��OH��2]��Ϊ�ⶨij��ʽ����ͭ����ɽ���������ʵ�飺ȡ�������ļ�ʽ����ͭ��Ʒ���ݣ�һ�ݵμ�ϡ������ǡ����ȫ�ܽ⣬��һ�ݸ������պ�ֻ�õ�CuO���塣����������ʾn��HCl���Un��CuO����3�U2����ü�ʽ����ͭ�Ļ�ѧʽ��x�Uy= ��
��3��E�Ƿǽ�������ǿ��Ԫ�أ�M��E����̬�⻯��ڹ̶�������ܱ������У�����M�������¹�ϵ��
xM(g) Mx(g)����Ӧ�������������ʵ�����ʱ��ı仯��ϵ����ͼ���� ��˵����ȷ����
Mx(g)����Ӧ�������������ʵ�����ʱ��ı仯��ϵ����ͼ���� ��˵����ȷ���� 
A���÷�Ӧ�Ļ�ѧ����ʽ��2HF (HF)2 (HF)2 |
| B��ƽ��ʱ��������ƽ��Ħ��������33.3 |
C��t1ʱ�̣������¶Ȳ��䣬�ٳ���1molM�����´ﵽƽ��ʱ�� ������ ������ |
| D��M�ķе��ͬ������һ����Ԫ�ص���̬�⻯��е�� |
��13 �֣�
��1��CuSO4��Һ����ѧ��ѧ����ũҵ�����г�����һ���Լ���ijͬѧ����CuSO4��Һ����������ʵ��̽����
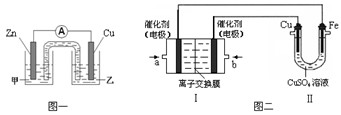
�� ͼһ�Ǹ��ݷ�ӦZn + CuSO4 = Cu + ZnSO4 ��Ƴɵ�пͭԭ��ء�����ʼ���Һ�� ���ZnSO4����CuSO4������Һ��Cu���ĵ缫��Ӧʽ�� ��
��ͼ���У����Ǽ���ȼ�ϵ�أ��������ҺΪKOH��Һ���Ľṹʾ��ͼ����ͬѧ���ڢ���ʵ�����϶�ͭ����b��ͨ����� ���CH4����O2������a���缫�Ϸ����ĵ缫��Ӧʽ�� ��
��2����������CuSO4��5H2O������ʯ�Һ�ˮ��һ��������ϣ����ɵõ�������Һ��ɱ���������� ��Ч�ɷ�Ϊ���ܵļ�ʽ����ͭ[xCuSO4 ��yCu��OH��2]��Ϊ�ⶨij��ʽ����ͭ����ɽ���������ʵ�飺ȡ�������ļ�ʽ����ͭ��Ʒ���ݣ�һ�ݵμ�ϡ������ǡ����ȫ�ܽ⣬��һ�ݸ������պ�ֻ�õ�CuO���塣����������ʾn��HCl���Un��CuO����3�U2����ü�ʽ����ͭ�Ļ�ѧʽ��x�Uy= ��
��3��E�Ƿǽ�������ǿ��Ԫ�أ�M��E����̬�⻯��ڹ̶�������ܱ������У�����M�������¹�ϵ��
xM(g) Mx
(g)����Ӧ�������������ʵ�����ʱ��ı仯��ϵ����ͼ���� ��˵����ȷ����
Mx
(g)����Ӧ�������������ʵ�����ʱ��ı仯��ϵ����ͼ���� ��˵����ȷ����
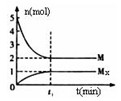
B��ƽ��ʱ��������ƽ��Ħ��������33.3
C��t1ʱ�̣������¶Ȳ��䣬�ٳ���1molM�����´ﵽƽ��ʱ�� ������
������
D��M�ķе��ͬ������һ����Ԫ�ص���̬�⻯��е��
 ������
������