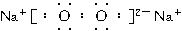��Ŀ����
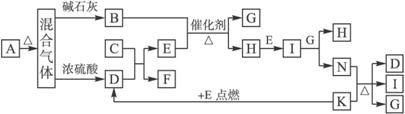
��ͼ��A��N�ֱ������ѧ��Ӧ�е�һ�ֳ������ʡ�A��һ�ֳ������ʣ�B�ڹ�ҵ�Ͽ���G�Ϳ����к������������Ƶá������£�C��Һ�塣E��N��HΪ�������ʣ�G��I��MΪ�ǽ������ʣ�����G��M�����塣K����Ȼ������Ҫ�ɷ֣�F�Ǿ��д��Եĺ�ɫ���塣����Ӧ���P������ȥ��

(1)F�Ļ�ѧʽΪ________��K���ӵĿռ�ṹ��________��
(2)��Ӧ�ݵ�������_________________________________��
(3)ʵ������ȡB�Ļ�ѧ����ʽ��______________________________��
(4)��Ӧ���У�����ȼ��H����ʢ��D �ļ���ƿ��ɹ۲쵽��������_____________________________________��
(5)д��ѧ����ʽ����Ӧ��_________________����Ӧ��____________________��
(6)��F����ϡ�����У����������ֲ�ͬ��̬�Ľ� �������ӡ�Ҫ�������еĸ�̬���������ӣ�Ӧ������Լ���___________________��
(2)��Ӧ�ݵ�������_________________________________��
(3)ʵ������ȡB�Ļ�ѧ����ʽ��______________________________��
(4)��Ӧ���У�����ȼ��H����ʢ��D �ļ���ƿ��ɹ۲쵽��������_____________________________________��
(5)д��ѧ����ʽ����Ӧ��_________________����Ӧ��____________________��
(6)��F����ϡ�����У����������ֲ�ͬ��̬�Ľ� �������ӡ�Ҫ�������еĸ�̬���������ӣ�Ӧ������Լ���___________________��
(1)Fe3O4����������
(2)ͨ��
(3)2NH4Cl��Ca(OH)2 CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2O
(4)þ���ڶ�����̼�о���ȼ�գ����ɰ�ɫ��ĩ��ƿ�ڱ��к�ɫ���帽��
(5)3Fe��4H2O(g) Fe3O4��4H2��3Fe3O4��8Al
Fe3O4��4H2��3Fe3O4��8Al 4Al2O3��9Fe
4Al2O3��9Fe
(6)KSCN��Һ
(2)ͨ��
(3)2NH4Cl��Ca(OH)2
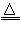 CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2O(4)þ���ڶ�����̼�о���ȼ�գ����ɰ�ɫ��ĩ��ƿ�ڱ��к�ɫ���帽��
(5)3Fe��4H2O(g)
 Fe3O4��4H2��3Fe3O4��8Al
Fe3O4��4H2��3Fe3O4��8Al 4Al2O3��9Fe
4Al2O3��9Fe(6)KSCN��Һ

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
�����Ŀ