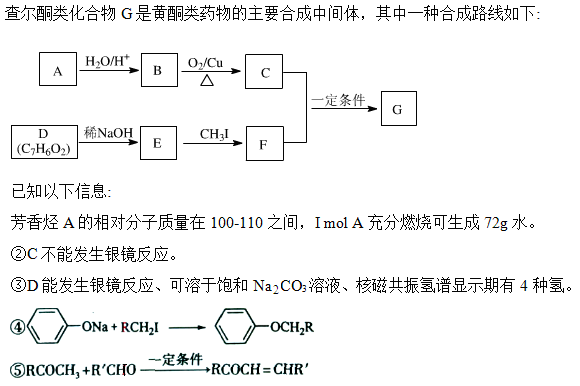
��Ŀ����
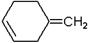
�ۼ���ϩ������ά�������ᡢƵ�ʿ������ԣ��㷺Ӧ�����������ά����֪AΪij�־ۼ���ϩ������ά�ĵ��壬��ת����ϵ���£�
��1��A�Ľṹ��ʽΪ ��
��2�������й�E��˵������ȷ���� ����д��ĸ��ţ�
��3��F��ͬ���칹��������������� ��
��4����Ӧ�ڵĻ�ѧ����ʽ��
��Ӧ�ݵĻ�ѧ����ʽ��

��1��A�Ľṹ��ʽΪ ��
��2�������й�E��˵������ȷ���� ����д��ĸ��ţ�
| A����ʹ���Ը��������Һ��ɫ | B����ʹ���CCl4��Һ��ɫ |
| C��һ�������£��ܹ�������ȥ��Ӧ | D��һ�������£��ܹ�����ȡ����Ӧ |
��4����Ӧ�ڵĻ�ѧ����ʽ��
��Ӧ�ݵĻ�ѧ����ʽ��
��1��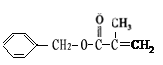
��2��C
��3��4
��4��2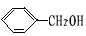 + O2
+ O2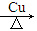 2 C6H5CHO��2H2O;
2 C6H5CHO��2H2O;
CH3CH2OH+CH3CH(CH3)COOH CH3CH(CH3)COOCH2CH3+ H2O
CH3CH(CH3)COOCH2CH3+ H2O
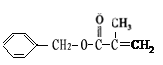
��2��C
��3��4
��4��2
 + O2
+ O2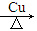 2 C6H5CHO��2H2O;
2 C6H5CHO��2H2O;CH3CH2OH+CH3CH(CH3)COOH
 CH3CH(CH3)COOCH2CH3+ H2O
CH3CH(CH3)COOCH2CH3+ H2O�����������1����A�����Ӿ۷�Ӧ�IJ����ж�A�Ľṹ��ʽΪ
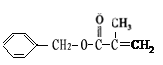 ��
����2��EΪ2-����ϩ�ᣬ����̼̼˫�����Է���ʹ���Ը��������Һ��ɫ�ķ�Ӧ��ʹ���CCl4��Һ��ɫ�ķ�Ӧ�������Ȼ����������ǻ�����ȡ����Ӧ�����ܷ�����ȥ��Ӧ����ѡC��
��3��E���������2-�����ᣬ���������ͬ���칹���м������������������������������������4�֣�
��4����Ӧ��Ϊ����������Ӧ����ѧ����ʽΪ2
 + O2
+ O2 2 C6H5CHO��2H2O��
2 C6H5CHO��2H2O����Ӧ����2-���������Ҵ���������Ӧ����ѧ����ʽ��
CH3CH2OH+CH3CH(CH3)COOH
 CH3CH(CH3)COOCH2CH3+ H2O��
CH3CH(CH3)COOCH2CH3+ H2O��
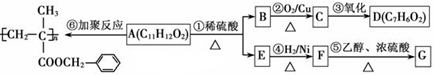
��ϰ��ϵ�д�
�����Ŀ
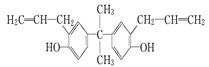
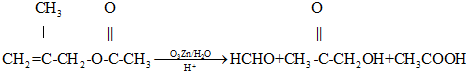
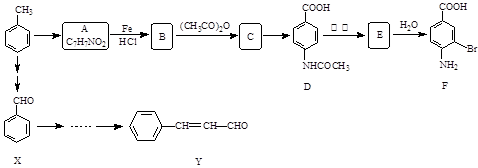
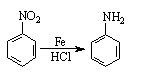 ������
������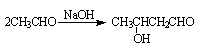 ��
��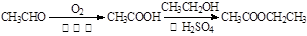
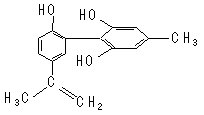
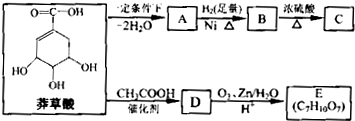
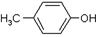 )���л���ҵ����Ҫԭ�ϣ������ںϳɶ����л��
)���л���ҵ����Ҫԭ�ϣ������ںϳɶ����л�� R��COOH��
R��COOH��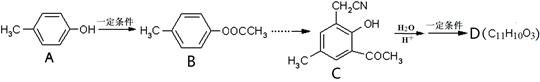
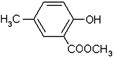 ���ϳ�·������ͼΪ��
���ϳ�·������ͼΪ��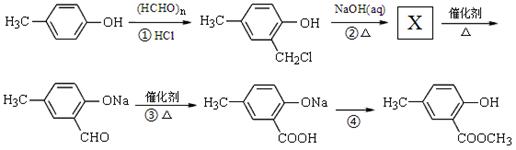
 �ĺϳ�·������ͼ��ʾ(�л����ýṹ��ʽ��ע����Ӧ�Լ�������)
�ĺϳ�·������ͼ��ʾ(�л����ýṹ��ʽ��ע����Ӧ�Լ�������) B����
B����