��Ŀ����
��12�֣����в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
��������������ش��������⣺(����ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
��1��A��B��C�ĵ縺����С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� �����⻯��ĵȵ������� ��
��3��E�ļ۵����Ų�ʽ�� ��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磬ECl3�γɵ������Ļ�ѧʽΪ ��
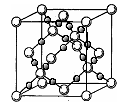
��4��AC2�ڸ��¸�ѹ�����γɵľ�����ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ������������壬�þ�����Aԭ�ӵ��ӻ���ʽΪ ��
��5��D �ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ�� ��
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ԭ�ӵĵ����Ų�ͼΪ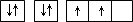 |
| B | �����µ���Ϊ˫ԭ�ӷ��ӣ�ԭ�Ӽ��γ����Թ��õ��Ӷ� |
| C | ԭ�ӵ�s�������������p�����������Ԫ�ص������Ϊ-2�� |
| D | ������������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ |
| E | ԭ��������D������ |
��1��A��B��C�ĵ縺����С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� �����⻯��ĵȵ������� ��
��3��E�ļ۵����Ų�ʽ�� ��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磬ECl3�γɵ������Ļ�ѧʽΪ ��
��4��AC2�ڸ��¸�ѹ�����γɵľ�����ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ������������壬�þ�����Aԭ�ӵ��ӻ���ʽΪ ��

��5��D �ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ�� ��
��1��C��N��O
��2�������� H3O+
��3��3d54s1 [Cr(NH3)4(H2O)2]Cl3
��4��ԭ�� sp3
��5�����Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ�(��)����ʽ�ͷ�����
��2�������� H3O+
��3��3d54s1 [Cr(NH3)4(H2O)2]Cl3
��4��ԭ�� sp3
��5�����Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ�(��)����ʽ�ͷ�����
�������֪��A��B��C��D��E��Ӧ��Ԫ�طֱ�Ϊ��C��N��O��Mg��Cr����Ԫ�أ�
��1��A��B��C�ĵ縺����С�����˳��Ϊ��C��N��O��������ͬ���ڴ������ң��縺��������
��2��B���⻯��ķ���ΪNH3����ռ�ṹΪ�����Σ���ȵ�������H3O+��
��3��Cr�ļ۵����Ų�ʽ�ǣ�3d54s1����CrCl3��CrԪ�صĻ��ϼ�Ϊ+3�ۣ���۵����Ų�ʽΪ��3d4����6���չ����3d��2���չ����4s��һ���չ����4p�������չ�������������Ļ�ѧʽΪ[Cr(NH3)4(H2O)2]Cl3��
��4����ͼ��֪�þ���ĽṹΪ�ռ���״�ṹ����������ԭ�Ӿ��壬�þ�����Aԭ�ӵ��ӻ���ʽΪsp3��
��5�����Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ�(��)����ʽ�ͷ�����
��1��A��B��C�ĵ縺����С�����˳��Ϊ��C��N��O��������ͬ���ڴ������ң��縺��������
��2��B���⻯��ķ���ΪNH3����ռ�ṹΪ�����Σ���ȵ�������H3O+��
��3��Cr�ļ۵����Ų�ʽ�ǣ�3d54s1����CrCl3��CrԪ�صĻ��ϼ�Ϊ+3�ۣ���۵����Ų�ʽΪ��3d4����6���չ����3d��2���չ����4s��һ���չ����4p�������չ�������������Ļ�ѧʽΪ[Cr(NH3)4(H2O)2]Cl3��
��4����ͼ��֪�þ���ĽṹΪ�ռ���״�ṹ����������ԭ�Ӿ��壬�þ�����Aԭ�ӵ��ӻ���ʽΪsp3��
��5�����Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ�(��)����ʽ�ͷ�����

��ϰ��ϵ�д�
�����Ŀ
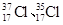 Ϊ��ͬ�ĺ��أ�
Ϊ��ͬ�ĺ��أ�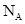 Ϊ�����ӵ�����������˵����ȷ����
Ϊ�����ӵ�����������˵����ȷ����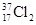 ����0.72
����0.72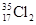 ��
��