��Ŀ����
����ԭ�ӽṹ��Ԫ�����ڱ���Ԫ�������ɵ�֪ʶ�ش��������⣺
(1)B��1��36��Ԫ��ԭ�Ӻ�������Ų���δ�ɶԵ���������Ԫ�أ�BԪ�ص�������____����Ԫ�����ڱ��е�λ����____________________��
(2)CԪ�ػ�̬ԭ�ӵĵ����Ų�ͼ����ͼ�е�_______(�����)����һ�����Ų�ͼ������Ϊ��̬ԭ�ӵĵ����Ų�ͼ����Ϊ��������_______(��A B C��ѡ��)��
A.�������ԭ�� B.����ԭ�� C.���ع���
��3�������������ϼ۴����͵���0��HԪ�ؿ��Է������ڱ��е� ��
(4)�Ȼ�淋ĵ���ʽ
(1)B��1��36��Ԫ��ԭ�Ӻ�������Ų���δ�ɶԵ���������Ԫ�أ�BԪ�ص�������____����Ԫ�����ڱ��е�λ����____________________��
(2)CԪ�ػ�̬ԭ�ӵĵ����Ų�ͼ����ͼ�е�_______(�����)����һ�����Ų�ͼ������Ϊ��̬ԭ�ӵĵ����Ų�ͼ����Ϊ��������_______(��A B C��ѡ��)��

A.�������ԭ�� B.����ԭ�� C.���ع���
��3�������������ϼ۴����͵���0��HԪ�ؿ��Է������ڱ��е� ��
(4)�Ȼ�淋ĵ���ʽ
��10�֣� (1) �� �������ڡ��ڢ�B��, (2) �ڡ�C (3) ��A ��4��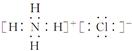
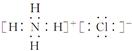
���������(1)���ݺ�����ӵ��Ų�ʽ��֪��1��36��Ԫ��ԭ�Ӻ�������Ų���δ�ɶԵ���������Ԫ���Ǹ���λ�ڵ������ڵڢ�B�塣
��2���ڵȼ۹��(ָ��ͬ���Ӳ㡢�����Dz��ϵĸ������)���Ų��ĵ��ӽ������ܷ�ռ��ͬ�Ĺ��,������������ͬ������Ǻ��ع�����Ȼ�ٲ����Ϻ��ع�����ȷ���Ų�ͼ�Ǣڡ�
��3�������������ϼ۴����͵���0��֪��HԪ�ص�����ǣ�1�ۡ���ͼ��ǣ�1�ۣ�������Ԫ�ؿ��Է������ڱ��еĢ�A�塣
��4���Ȼ���Ǻ������Ӽ������ӻ��������ʽ��
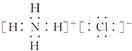 ��
�������������ǻ���������Ŀ��飬Ҳ�Ǹ߿��еij������㣬�ѶȲ������������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ����ּ�ڿ���ѧ��������û�����֪ʶ���ʵ�����������������������ѧ���������������ͷ�ɢ˼ά������

��ϰ��ϵ�д�
�����Ŀ

