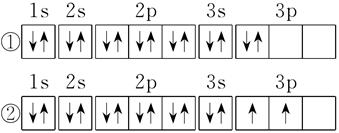
ƒøƒ⁄»ð
‘™Àÿ÷Ð∆⁄±Ì”Α™Àÿ÷Ð∆⁄¬…‘⁄—ßœ∞°¢—–æø∫Õ…˙≤˙ µº˘÷–”–∫Ð÷ÿ“™µƒ◊˜”√°£œ¬±Ì¡–≥ˆ¡À¢Ÿ°´¢·æ≈÷÷‘™Àÿ‘⁄÷Ð∆⁄±Ì÷–µƒŒª÷√°£«Îªÿ¥£∫
(1)’‚æ≈÷÷‘™Àÿ÷–(ÃÓ‘™Àÿ∑˚∫≈)¢ð « £¨∆‰÷–ªØ—ß–‘÷ ◊Ó≤ªªÓ∆√µƒ « °£
¢ð∫Õ¢Þ–Œ≥…ªØ∫œŒÔµƒµÁ◊” Ω
(2)‘⁄’‚–©‘™Àÿµƒ◊Ó∏þº€—ıªØŒÔ∂‘”¶µƒÀƪ،Ô÷–£¨ºÓ–‘◊Ó«øµƒ «(Ãѧ Ω)°£
À·–‘◊Ó«øµƒ « ≥ ¡Ω–‘µƒ « £¨–¥≥ˆ»˝’þ÷ƺ‰œýª•∑¥”¶µƒªØ—ß∑Ω≥Ã Ω £ª
(3)¢Ÿ°¢¢⁄°¢¢€»˝÷÷‘™Àÿ∞¥‘≠◊”∞Îæ∂”…¥ÛµΩ–°µƒÀ≥–Ú“¿¥ŒŒ™ (ÃÓ‘™Àÿ∑˚∫≈)°£
(4) ”√µÁ◊” Ω±Ì æ¢ý‘™Àÿ”΢€‘™Àÿ–Œ≥…ªØ∫œŒÔµƒπ˝≥à °£
(5) ‘⁄¢Ÿ∫Õ¢⁄÷–ªØ—ß–‘÷ ◊ÓªÓ∆√µƒ «
(6)‘⁄¢þ∫Õ¢ý÷–ªØ—ß–‘÷ ◊ÓªÓ∆√µƒ « £¨”√ªØ—ß µ—È÷§√˜µƒ∑Ω∑®∫Õ¿Î◊”∑Ω≥Ã Ω£∫
∑Ω∑®
¿Î◊”∑Ω≥à Ω
| ◊ ÷Ð∆⁄ | ¢ÒA | ¢ÚA | ¢ÛA | ¢ÙA | ¢ıA | ¢ˆA | ¢˜A | 0 |
| 2 | | | | ¢ð | | ¢Þ | | |
| 3 | ¢Ÿ | ¢€ | ¢Ð | | | | ¢þ | ¢· |
| 4 | ¢⁄ | | | | | | ¢ý | |
¢ð∫Õ¢Þ–Œ≥…ªØ∫œŒÔµƒµÁ◊” Ω
(2)‘⁄’‚–©‘™Àÿµƒ◊Ó∏þº€—ıªØŒÔ∂‘”¶µƒÀƪ،Ô÷–£¨ºÓ–‘◊Ó«øµƒ «(Ãѧ Ω)°£
À·–‘◊Ó«øµƒ « ≥ ¡Ω–‘µƒ « £¨–¥≥ˆ»˝’þ÷ƺ‰œýª•∑¥”¶µƒªØ—ß∑Ω≥Ã Ω £ª
(3)¢Ÿ°¢¢⁄°¢¢€»˝÷÷‘™Àÿ∞¥‘≠◊”∞Îæ∂”…¥ÛµΩ–°µƒÀ≥–Ú“¿¥ŒŒ™ (ÃÓ‘™Àÿ∑˚∫≈)°£
(4) ”√µÁ◊” Ω±Ì æ¢ý‘™Àÿ”΢€‘™Àÿ–Œ≥…ªØ∫œŒÔµƒπ˝≥à °£
(5) ‘⁄¢Ÿ∫Õ¢⁄÷–ªØ—ß–‘÷ ◊ÓªÓ∆√µƒ «
(6)‘⁄¢þ∫Õ¢ý÷–ªØ—ß–‘÷ ◊ÓªÓ∆√µƒ « £¨”√ªØ—ß µ—È÷§√˜µƒ∑Ω∑®∫Õ¿Î◊”∑Ω≥Ã Ω£∫
∑Ω∑®
¿Î◊”∑Ω≥à Ω
£®1£©C Ar 
£®2£© KOH HClO4 Al(OH)3 KOH +HClO4£ΩKClO4 +H2O KOH + Al(OH)3£ΩKAlO2 +2H2O
(3) K£æNa£æMg (4)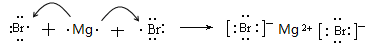 £®5£©K
£®5£©K
(6) Cl »° ¡ø‰ÂªØƒ∆»Ð“∫µŒ»Î¬»ÀÆ£¨»Ð“∫±‰Œ™≥»ª∆…´£¨‘Ú∑«Ω Ù–‘Cl£æBr Cl2 + 2Br-£Ω 2Cl- + Br2

£®2£© KOH HClO4 Al(OH)3 KOH +HClO4£ΩKClO4 +H2O KOH + Al(OH)3£ΩKAlO2 +2H2O
(3) K£æNa£æMg (4)
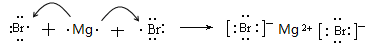 £®5£©K
£®5£©K (6) Cl »° ¡ø‰ÂªØƒ∆»Ð“∫µŒ»Î¬»ÀÆ£¨»Ð“∫±‰Œ™≥»ª∆…´£¨‘Ú∑«Ω Ù–‘Cl£æBr Cl2 + 2Br-£Ω 2Cl- + Br2
‘Â∑÷Œˆ£∫£®1£©∏˘æð‘™Àÿ‘⁄÷Ð∆⁄±Ì÷–µƒœý∂‘Œª÷√ø…÷™¢Ÿ°´¢·æ≈÷÷‘™Àÿ∑÷± «Na°¢K°¢Mg°¢Al°¢C°¢O°¢Cl°¢Br°¢Ar°£Ar «œ°”–∆¯Ã‘™Àÿ£¨ªØ—ß–‘÷ ◊Ó≤ªªÓ∆√°£¢ð∫Õ¢Þ–Œ≥…ªØ∫œŒÔ «CO2£¨∫¨”–π≤º€º¸µƒπ≤º€ªØ∫œŒÔ£¨∆‰µÁ◊” Ω «
 °£
°££®2£©Ω Ù–‘ªÚ∑«Ω Ù–‘‘Ω«ø£¨◊Ó∏þº€—ıªØŒÔµƒÀƪ،ԵƒºÓ–‘ªÚÀ·–‘‘Ω«ø£¨‘ÚKOHµƒºÓ–‘◊Ó«ø£¨HClO4µƒÀ·–‘◊Ó«ø£¨ Al(OH)3 «¡Ω–‘«‚—ıªØŒÔ°£
£®3£©Õ¨÷Ð∆⁄◊‘◊ۜڔ“‘≠◊”∞Îæ∂÷Ω•ºı–°£¨Õ¨÷˜◊Â◊‘…œ∂¯œ¬‘≠◊”∞Îæ∂÷Ω•‘ˆ¥Û£¨‘Ú»˝÷÷‘™Àÿµƒ‘≠◊”∞Îæ∂¥Û–°À≥–Ú «K£æNa£æMg°£
£®4£© æ¢ý‘™Àÿ”΢€‘™Àÿ–Œ≥…ªØ∫œŒÔ «‰ÂªØ√棨∫¨”–¿Î◊”º¸£¨∆‰–Œ≥…π˝≥Ãø…±Ì 挙
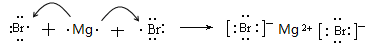 °£
°££®5£©Õ¨÷˜◊Â◊‘…œ∂¯œ¬£¨Ω Ù–‘÷Ω•‘ˆ«ø£¨À˘“‘KµƒΩ Ù–‘«ø”⁄NaµƒΩ Ù–‘°£
£®6£©Õ¨÷˜◊Â◊‘…œ∂¯œ¬£¨∑«Ω Ù–‘÷Ω•ºı»ı£¨À˘“‘Clµƒ∑«Ω Ù–‘«ø”⁄Brµƒ∑«Ω Ù–‘°£ø…∏˘æð∑«Ω Ù–‘«øµƒµ•÷ ◊™ªª»ı£¨º¥»° ¡ø‰ÂªØƒ∆»Ð“∫µŒ»Î¬»ÀÆ£¨»Ð“∫±‰Œ™≥»ª∆…´£¨‘Ú∑«Ω Ù–‘Cl£æBr£¨À˘“‘µƒ¿Î◊”∑Ω≥Ã Ω «Cl2 + 2Br-£Ω2Cl- + Br2°£
µ„∆¿£∫∏√ «ª˘¥°–‘ ‘µƒøº≤È£¨≤ý÷ÿ∂‘—ß…˙ª˘¥°÷™ ∂πÆπÃ∫Õ—µ¡∑µƒÕ¨ ±£¨÷º‘⁄øº≤È—ß…˙¡ÈªÓ‘À”√ª˘¥°÷™ ∂Ω‚æˆ µº Œ µƒƒÐ¡¶£¨”–¿˚”⁄≈ý—¯—ß…˙µƒ¬þº≠Õ∆¿ÌƒÐ¡¶∫ÕπÊ∑∂¥Ã‚ƒÐ¡¶°£

¡∑œ∞≤·œµ¡–¥∞∏
œýπÿƒø
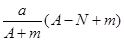 mol
mol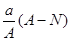 mol
mol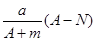 mol
mol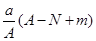 mol
mol