��Ŀ����
19���ں���ʱ����ij�ܱ�������ͨ��2mol X��1mol Y���壬�������·�Ӧ��2X ������+Y������?2Z��������ѹǿһ��ʱ�������ƽ��ʱZ���������Ϊ0.4����1��������ƽ�Ᵽ��ͬ�¡�ͬѹ�������ܱ�������ͨ��4mol X��������2mol Y���������ﵽƽ�⣬��Z���������Ϊ40%��ƽ��ʱ������������ʵ�����5mol��
��2�������ܱ�����ͨ��X��������Y�����������ʵ����ֱ��Ϊn��X����n��Y�������������ݻ����䣬������ƽ��ʱZ���������Ϊ0.4����n��X��/n��Y����ȡֵ��ΧΪ0.5��$\frac{n��X��}{n��Y��}$��5��
���� ��1����ԭƽ��Ϊ��Чƽ�⣬����ֵĺ�����ԭƽ���Ӧ��ͬ����������ʽ���ⷨ���㣮����Z���������Ϊ0.4�з��̣���⣮
��2������Z���������Ϊ0.4�з��̣���Ӧ�ﲻ����ȫ��Ӧ��ƽ��ʱ���㣬�в���ʽ��������⣮
��� �⣺��1������ʱ���ܱ�������ͨ��2molX��g����1molY��g�� ������Ӧ��ƽ�⣬ѹǿһ��ʱZ���������Ϊ0.4��������ƽ�Ᵽ��ͬ�¡�ͬѹ����n ��X����n ��Y��=2��1��
��Ϊ��Чƽ�⣬Z�����������Ϊ0.4��������Y���ʵ���Ϊx��
2X��g��+Y��g��?2Z��g����
��ʼ���ʵ�����mol����4 2 0
�仯���ʵ�����mol�� 2x x 2x
ƽ�����ʵ�����mol��4-2x 2-x 2x
����$\frac{2x}{��4-2x+2-x+2x��}$��100%=40%�����x=1��
n���ܣ�=4-2x+2-x+2x=6-x=5mol��
�ʴ�Ϊ��40%��5mol��
��2���跴Ӧ������Y��������Ϊy��
2X��g��+Y��g��?2Z��g����
��ʼ���ʵ�����mol���� n��X�� n��Y�� 0
�仯���ʵ�����mol�� 2y y 2y
ƽ�����ʵ�����mol��n��X��-2y n��Y��-y 2y
������$\frac{2y}{n��X��-2y+n��Y��+2y}$��100%=40%��
���n��X��+n��Y��=6y���٣���n��X��-2y��0���ڣ�n��Y��-y��0���ۣ�
�����٢۽�ã�n��X��+n��Y����6n��Y����n��X����5n��Y����
�����٢ڵõ���n��X��+n��Y����$\frac{1}{2}$��6n��Y����n��X����0.5n��Y����
�õ���0.5n��Y����n��X����5n��Y��
�ʴ�Ϊ��0.5��$\frac{n��X��}{n��Y��}$��5��
���� ���⿼���Чƽ��ļ��㣬��Ŀ�ѶȽϴ�ע��ȷ�����Чƽ���ԭ����Ӧ�ã�

| A�� | �ü�������ͭ�۵ķ�����ȥCu��NO3��2��Һ�л��е�AgNO3 | |
| B�� | ��ϴ��ƿ�е�NaOH��Һ��ȥCO2�л��е�HCl���� | |
| C�� | ��ij��ɫδ֪��Һ�н�����BaCl2��Һ���Լ���δ֪��Һ��SO42- | |
| D�� | �Ⱥ����ӷ�̪��Һ��BaCl2��Һ�������ᡢ���ᡢ�����ơ��������ƺ������������ɫ��Һ���� |
| A�� | �����ȷ�Ӧ | B�� | �Ƿ��ȷ�Ӧ | ||
| C�� | ���ؼ�С�ķ�Ӧ | D�� | ����ЧӦ������ЧӦ |
| A�� | �����£�����Ƭ����Ũ�����У�Fe+6HNO3��Ũ���TFe��NO3��3+3NO2��+3H2O | |
| B�� | ���Ȼ�淋�ϡ��Һ�м�������NaOH��NH4++OH-�TNH3��+H2O | |
| C�� | ��NH4��2SO4��Һ�м�������Ba��OH��2��Ba2++SO42-�TBaSO4�� | |
| D�� | ��Ũ�����м���ͭƬ��Cu+4H++2NO3-�TCu2++2NO2��+2H2O |
| A�� | ����һ����ɫ��ζ������ | B�� | ���������ȶ��������������ʷ�Ӧ | ||
| C�� | ���鼫���ܽ���ˮ | D�� | �����ܶȱȿ������ܶ�С |
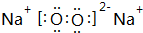 ������������ˮ��Ӧ�����ӷ���ʽ2Na2O2+2H2O=4Na++4OH-+O2����
������������ˮ��Ӧ�����ӷ���ʽ2Na2O2+2H2O=4Na++4OH-+O2����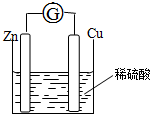 ��пƬ��ͭƬ�õ�����������ϡ���������ԭ��أ����缫������һ�������ƣ�װ����ͼ��
��пƬ��ͭƬ�õ�����������ϡ���������ԭ��أ����缫������һ�������ƣ�װ����ͼ��