��Ŀ����
������25mL��Ũ�ȵ������зֱ�����������ȵ�NaHCO3��KHCO3�Ļ�����ò������������������������ܽ⣩�������ʾ������˵����ȷ����
| ��� | 1 | 2 | 3 |
| m������ | 4.6g | 7.2g | 7.9g |
| V��CO2������״���� | 1.12L | 1.68L | 1.68L |
- A.���ݵ�1�����ݿ��Լ���������Ũ��
- B.�������NaHCO3����������ԼΪ45.7%
- C.���ݵ�2��3�����ݲ��ܷ�������2��Ļ�����Ƿ���ȫ��Ӧ
- D.��������ʵ���Ũ��Ϊ1.0 mol?L-1
BC
������A��������Ũ����ѡ��������������ݽ��У������Ż�����������ӣ�������̼�������������ӣ���������ȫ����Ӧ�ꣻ
B��3��ʵ����������ͬ������������ͬ���ɱ������ݿ�֪����1�����������С���ʼ���4.6g�����ʱ��������ʣ�࣬�������ȫ��Ӧ����������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol�����ݹ����������������������״���µĶ�����̼���������з��̼���x��y���ٸ���m=nM����NaHCO3����������������NaHCO3������������
C�����ݵ�2��3�����ݿ�֪��������������ȣ����ɶ�����̼�������ȣ�˵����2��3��ʵ���������꣬��3��ʵ���й���һ����ʣ�࣬���ݵ�2��3�����ݲ���ȷ����2��Ļ�����Ƿ���ȫ��Ӧ�����Խ�����1�����ݣ����ݹ�������������ı�����ϵ�������1.68L������̼����������������жϣ�
D��������Ũ����ѡ��������������ݽ��У������Ż�����������ӣ�������̼�������������ӣ���������ȫ����Ӧ�꣬�ɱ��е�2��3�����ݿ�֪��������������ȣ����ɶ�����̼�������ȣ�˵����2��3��ʵ����������ȫ��Ӧ���ɶ�����̼1.68L�����H++HCO-3=H2O+CO2������n��HCl����������c=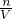 ���㣮
���㣮
���A��������Ũ����ѡ��������������ݽ��У�3��ʵ����������ͬ������������ͬ���ɱ������ݿ�֪����1�����������С���ʼ���4.6g�����ʱ��������ʣ�࣬�ʵ�1�����ݲ��ܼ��������Ũ�ȣ���A����
B��3��ʵ����������ͬ������������ͬ���ɱ������ݿ�֪����1�����������С���ʼ���4.6g�����ʱ��������ʣ�࣬�������ȫ��Ӧ����������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol����84x+100y=4.6��x+y= �����x=0.025��y=0.025����NaHCO3������Ϊ0.025mol��84g/mol=2.1g��NaHCO3����������Ϊ
�����x=0.025��y=0.025����NaHCO3������Ϊ0.025mol��84g/mol=2.1g��NaHCO3����������Ϊ ��100%=45.7%����B��ȷ��
��100%=45.7%����B��ȷ��
C�����ݵ�2��3�����ݿ�֪��������������ȣ����ɶ�����̼�������ȣ�˵����2��3��ʵ���������꣬��3��ʵ���й���һ����ʣ�࣬���ݵ�2��3�����ݲ���ȷ����2��Ļ�����Ƿ���ȫ��Ӧ�����Խ�����1�����ݣ����ݹ�������������ı�����ϵ�������1.68L������̼����������������жϣ���C��ȷ��
D��������Ũ����ѡ��������������ݽ��У������Ż�����������ӣ�������̼�������������ӣ���������ȫ����Ӧ�꣬�ɱ��е�2��3�����ݿ�֪��������������ȣ����ɶ�����̼�������ȣ�˵����2��3��ʵ����������ȫ��Ӧ���ɶ�����̼1.68L����H++HCO-3=H2O+CO2����֪n��HCl��=n��CO2��= =0.075mol�������Ũ��Ϊ
=0.075mol�������Ũ��Ϊ =3mol/L����D����
=3mol/L����D����
��ѡBC��
���������⿼��������йؼ��㣬�Ѷ��еȣ����ݶ�����̼������仯�ж������Ƿ���ȫ��Ӧʽ�ǹؼ����ٸ���������������������ϵ���н��
������A��������Ũ����ѡ��������������ݽ��У������Ż�����������ӣ�������̼�������������ӣ���������ȫ����Ӧ�ꣻ
B��3��ʵ����������ͬ������������ͬ���ɱ������ݿ�֪����1�����������С���ʼ���4.6g�����ʱ��������ʣ�࣬�������ȫ��Ӧ����������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol�����ݹ����������������������״���µĶ�����̼���������з��̼���x��y���ٸ���m=nM����NaHCO3����������������NaHCO3������������
C�����ݵ�2��3�����ݿ�֪��������������ȣ����ɶ�����̼�������ȣ�˵����2��3��ʵ���������꣬��3��ʵ���й���һ����ʣ�࣬���ݵ�2��3�����ݲ���ȷ����2��Ļ�����Ƿ���ȫ��Ӧ�����Խ�����1�����ݣ����ݹ�������������ı�����ϵ�������1.68L������̼����������������жϣ�
D��������Ũ����ѡ��������������ݽ��У������Ż�����������ӣ�������̼�������������ӣ���������ȫ����Ӧ�꣬�ɱ��е�2��3�����ݿ�֪��������������ȣ����ɶ�����̼�������ȣ�˵����2��3��ʵ����������ȫ��Ӧ���ɶ�����̼1.68L�����H++HCO-3=H2O+CO2������n��HCl����������c=
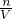 ���㣮
���㣮���A��������Ũ����ѡ��������������ݽ��У�3��ʵ����������ͬ������������ͬ���ɱ������ݿ�֪����1�����������С���ʼ���4.6g�����ʱ��������ʣ�࣬�ʵ�1�����ݲ��ܼ��������Ũ�ȣ���A����
B��3��ʵ����������ͬ������������ͬ���ɱ������ݿ�֪����1�����������С���ʼ���4.6g�����ʱ��������ʣ�࣬�������ȫ��Ӧ����������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol����84x+100y=4.6��x+y=
 �����x=0.025��y=0.025����NaHCO3������Ϊ0.025mol��84g/mol=2.1g��NaHCO3����������Ϊ
�����x=0.025��y=0.025����NaHCO3������Ϊ0.025mol��84g/mol=2.1g��NaHCO3����������Ϊ ��100%=45.7%����B��ȷ��
��100%=45.7%����B��ȷ��C�����ݵ�2��3�����ݿ�֪��������������ȣ����ɶ�����̼�������ȣ�˵����2��3��ʵ���������꣬��3��ʵ���й���һ����ʣ�࣬���ݵ�2��3�����ݲ���ȷ����2��Ļ�����Ƿ���ȫ��Ӧ�����Խ�����1�����ݣ����ݹ�������������ı�����ϵ�������1.68L������̼����������������жϣ���C��ȷ��
D��������Ũ����ѡ��������������ݽ��У������Ż�����������ӣ�������̼�������������ӣ���������ȫ����Ӧ�꣬�ɱ��е�2��3�����ݿ�֪��������������ȣ����ɶ�����̼�������ȣ�˵����2��3��ʵ����������ȫ��Ӧ���ɶ�����̼1.68L����H++HCO-3=H2O+CO2����֪n��HCl��=n��CO2��=
 =0.075mol�������Ũ��Ϊ
=0.075mol�������Ũ��Ϊ =3mol/L����D����
=3mol/L����D������ѡBC��
���������⿼��������йؼ��㣬�Ѷ��еȣ����ݶ�����̼������仯�ж������Ƿ���ȫ��Ӧʽ�ǹؼ����ٸ���������������������ϵ���н��

��ϰ��ϵ�д�
�����Ŀ
������25mL��Ũ�ȵ������зֱ�����������ȵ�NaHCO3��KHCO3�Ļ�����ò������������������������ܽ⣩�������ʾ������˵����ȷ���ǣ�������
|
������25mL��Ũ�ȵ������зֱ�����������ȵ�NaHCO3��KHCO3�Ļ�����ò������������������������ܽ⣩�������ʾ������˵����ȷ���ǣ� ��
A�����ݵ�1�����ݿ��Լ���������Ũ��
B���������NaHCO3����������ԼΪ45.7%
C�����ݵ�2��3�����ݲ��ܷ�������2��Ļ�����Ƿ���ȫ��Ӧ
D����������ʵ���Ũ��Ϊ1��O mol?L-1
| ��� | 1 | 2 | 3 |
| m������ | 4.6g | 7.2g | 7.9g |
| V��CO2������״���� | 1.12L | 1.68L | 1.68L |
A�����ݵ�1�����ݿ��Լ���������Ũ��
B���������NaHCO3����������ԼΪ45.7%
C�����ݵ�2��3�����ݲ��ܷ�������2��Ļ�����Ƿ���ȫ��Ӧ
D����������ʵ���Ũ��Ϊ1��O mol?L-1