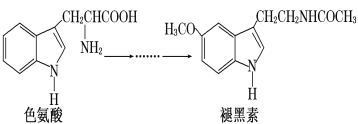
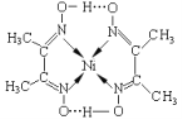
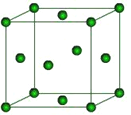
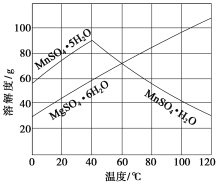
��Ŀ����
����Ŀ�������е�H2Sͨ�������ȷֽ����ȡ������2H2S(g)![]() 2H2(g)��S2(g)������3L�ܱ������У����Ʋ�ͬ�¶Ƚ���H2S�ֽ�ʵ�顣
2H2(g)��S2(g)������3L�ܱ������У����Ʋ�ͬ�¶Ƚ���H2S�ֽ�ʵ�顣
��1��ij�¶�ʱ����÷�Ӧ��ϵ��������1.3lmol����Ӧ1 min�������Ϊl.37mol,��tmin ��H2����������Ϊ___________��
��2��ij�¶�ʱ��H2S��ת���ʴﵽ���ֵ��������_____________(ѡ����)��
a�������ѹǿ�������仯 b��������ܶȲ������仯
c��![]() �������仯 d����λʱ����ֽ��H2S�����ɵ�H2һ����
�������仯 d����λʱ����ֽ��H2S�����ɵ�H2һ����
��3��ʵ��������ͼ��ͼ������a��ʾH2S��ƽ��ת�������¶ȹ�ϵ������b��ʾ��ͬ�¶��¡���Ӧ������ͬʱ����δ�ﵽ��ѧƽ��ʱH2S��ת���ʡ��÷�ӦΪ_____��Ӧ������ȡ������ȡ���������b���¶ȵ����ߣ�������aͨ����ԭ����_________��������������������£����Ҫ���H2������������ɲ�ȡ��һ�ִ�ʩ��________��

��4��ʹ1LH2S��20L������������O2�������Ϊ0.2����ȫ��Ӧ��ָ������£��������������______L ����2gH2S��ȫȼ�պ����ɶ��������ˮ������ͬʱ�ų�29.4 kJ���������÷�Ӧ���Ȼ�ѧ����ʽ��__________________��
���𰸡� 0.04/t mol/(L��min) a��c ���� �����¶����ߣ���Ӧ���ʼӿ죬�ﵽƽ������Ҫ��ʱ���� ���߷�Ӧ�¶ȣ���ʱ����S2����ȣ���дһ�������������� 19.5 2H2S(g)+3O2(g)��2SO2(g)+2H2O(g)+999.6kJ
����������1�����ݷ���ʽ��֪ÿ����2mol�������������1L���¶�ʱ����÷�Ӧ��ϵ��������1.3lmol����Ӧ1 min�������Ϊl.37mol���������0.06mol����������������0.12mol��Ũ����0.04mol/L����tmin ��H2����������Ϊ0.04/t mol/(L��min)����2��ij�¶�ʱ��H2S��ת���ʴﵽ���ֵ��˵���ﵽƽ��״̬��a������Ӧ������ӣ��������ѹǿ�������仯˵���ﵽƽ��״̬��a��ȷ��b���ܶ��ǻ������������������ݻ��ı�ֵ���������ݻ������䣬���������ܶȲ������仯����˵���ﵽƽ��״̬��b����c��![]() ��ʾƽ�ⳣ���������˵���ﵽƽ��״̬��c��ȷ��d����λʱ����ֽ��H2S�����ɵ�H2һ�������ʾ���淴Ӧ���ʣ�����˵���ﵽƽ��״̬��d����ѡac����3������ͼ���֪�����¶�ת��������˵�������¶�ƽ��������Ӧ������У���˸÷�ӦΪ���ȷ�Ӧ�����������¶����ߣ���Ӧ���ʼӿ죬�ﵽƽ������Ҫ��ʱ���̣���������b���¶ȵ����ߣ�������a����������Ӧ������������ȵĿ��淴Ӧ�����������������������£����Ҫ���H2������������ɲ�ȡ�Ĵ�ʩ�����߷�Ӧ�¶ȣ���ʱ����S2����ȡ���4��20L�����������������20L��0.2=4L��������H2S��Ӧ�ķ���ʽΪ2H2S+3O2��2SO2+2H2O����˵������������ʣ��������4L��1.5L��2.5L������SO2��1L������ȫ��Ӧ��ָ������£��������������16L��2.5L��1L��19.5L����2gH2S��ȫȼ�պ����ɶ��������ˮ������ͬʱ�ų�29.4 kJ����������2molH2S��ȫ��Ӧ�ų���������
��ʾƽ�ⳣ���������˵���ﵽƽ��״̬��c��ȷ��d����λʱ����ֽ��H2S�����ɵ�H2һ�������ʾ���淴Ӧ���ʣ�����˵���ﵽƽ��״̬��d����ѡac����3������ͼ���֪�����¶�ת��������˵�������¶�ƽ��������Ӧ������У���˸÷�ӦΪ���ȷ�Ӧ�����������¶����ߣ���Ӧ���ʼӿ죬�ﵽƽ������Ҫ��ʱ���̣���������b���¶ȵ����ߣ�������a����������Ӧ������������ȵĿ��淴Ӧ�����������������������£����Ҫ���H2������������ɲ�ȡ�Ĵ�ʩ�����߷�Ӧ�¶ȣ���ʱ����S2����ȡ���4��20L�����������������20L��0.2=4L��������H2S��Ӧ�ķ���ʽΪ2H2S+3O2��2SO2+2H2O����˵������������ʣ��������4L��1.5L��2.5L������SO2��1L������ȫ��Ӧ��ָ������£��������������16L��2.5L��1L��19.5L����2gH2S��ȫȼ�պ����ɶ��������ˮ������ͬʱ�ų�29.4 kJ����������2molH2S��ȫ��Ӧ�ų���������![]() ����˸÷�Ӧ���Ȼ�ѧ����ʽ�� 2H2S(g)+3O2(g)��2SO2(g)+2H2O(g)+999.6kJ��
����˸÷�Ӧ���Ȼ�ѧ����ʽ�� 2H2S(g)+3O2(g)��2SO2(g)+2H2O(g)+999.6kJ��