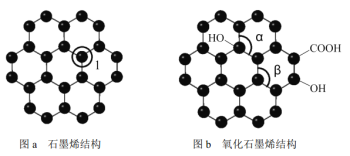
��Ŀ����
����Ŀ����A��B��C��D����Ԫ�أ����ǵ�ԭ����������������С��18��A��B��ͬһ���ڣ�A�ĵ���ʽΪ![]() ��Bԭ��L��ĵ���������K���3����0.1 mol C���ʴ������û���2.24 L����(��״��)��ͬʱ�����Neԭ�ӵĵ��Ӳ�ṹ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
��Bԭ��L��ĵ���������K���3����0.1 mol C���ʴ������û���2.24 L����(��״��)��ͬʱ�����Neԭ�ӵĵ��Ӳ�ṹ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
(1)д��A��B��C��DԪ�ص����ƣ�A______��B______��C______��D______��
(2)DԪ�������ڱ��е�______���ڵ�______�壻
(3)�õ���ʽ��ʾA����̬�⻯����γɹ���_______________________��
(4)A��B�ĵ��ʳ�ַ�Ӧ���ɵĻ�����Ľṹʽ��__________________��
(5)����Ԫ���У�______������������ˮ������������ᣬ�������ڼ����NaOH��Һ�Ļ�ѧ����ʽΪ________________��
���𰸡�̼ �� þ �� �� ��A  O=C=O Al Al(OH)3+NaOH= NaAlO2+2H2O
O=C=O Al Al(OH)3+NaOH= NaAlO2+2H2O
��������
Bԭ��L��ĵ���������K���3��������L����6�����ӣ�����BΪOԪ�أ�A��Bλ��ͬһ���ڣ���A�������4�����ӣ���AΪCԪ�أ�0.1 mol C���ʴ������û���2.24 L����(��״��)����0.1mol������ͬʱ�����Neԭ�ӵĵ��Ӳ�ṹ����C��������Ӧ��������λ����ɣ�����ΪMgԪ�أ�D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ����Dֻ����λ��Mg֮���ͬ���ڽ���Ԫ�أ���AlԪ�ء�
(1)���ݷ�����֪AΪ̼��BΪ����CΪþ��DΪ����
(2)��ԭ�ӵĺ˵����Ϊ13��λ��Ԫ�����ڱ��ĵ������ڵ���A�壻
(3)A����̬�⻯��ΪCH4�����ڹ��ۻ�����õ���ʽ��ʾ�γɹ��̣�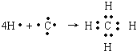 ��
��
(4)̼���ʺ�������ַ�Ӧ���ɶ�����̼�����ڹ��ۻ�����ṹʽΪO=C=O��
(5)��Ԫ�ص�����������ˮ����Al(OH)3�����������ᣬ�������ڼ����NaOH��Һ����ƫ�����ƺ�ˮ������ʽΪAl(OH)3+NaOH= NaAlO2+2H2O��

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�����Ŀ����¯���������з�������Ҫ��ӦΪ��![]() Fe2O3(s) + CO(g)
Fe2O3(s) + CO(g) ![]()
![]() Fe(s) + CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
Fe(s) + CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
�¶�/�� | 1000 | 1150 | 1300 |
ƽ�ⳣ�� | 4.0 | 3.7 | 3.5 |
��ش��������⣺
��1���÷�Ӧ��ƽ�ⳣ������ʽK=_____________����H________0(����>������<������=��)��
��2����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe2O3��CO��1.0 mol����Ӧ����l0 min��ﵽƽ�⡣���ʱ�䷶Χ�ڷ�Ӧ��ƽ����Ӧ������(CO2)=______________________��CO��ƽ��ת����= _____________��
��3������ߣ�2����CO��ƽ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________��
A������Fe���� B������Fe2O3���� C���Ƴ�����CO2
D����߷�Ӧ�¶� E����С�������ݻ� F��������ʵĴ���
��4����1L���ܱ������У�1300�����������д�ƽ��״̬����_______________��
A | B | C | D | |
n(Fe2O3) | 0.350 | 0.027 | 0.080 | 0.080 |
n(CO) | 0.010 | 0.010 | 0.010 | 0.050 |
n(Fe) | 0.100 | 0.064 | 0.080 | 0.080 |
n(CO2) | 0.035 | 0.088 | 0.040 | 0.050 |
.