��Ŀ����
����Ŀ��ʵ���ҳ���Ũ����Ͷ������̷�Ӧ��ȡ���������������ø��������������
��1��ijѧ������������ʵ����Ʒ��
ʵ������������̨���ƾ��ơ���Ȧ�����С�Բ����ƿ��˫����������Һ©�����������ܡ���Ƥ�ܡ�ϴ��ƿ��2����������ƿ�����ȱ�ٵ�������________��_________��
�Լ����������̡�Ũ���ᡢ����������Һ����ȱ�ٵ��Լ���__________��
��2��д����ʵ������ȡ������Ӧ�Ļ�ѧ����ʽ______________________________��
��3�������Լ����������Ƶ�������______________________________��
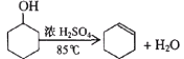
���𰸡�ʯ�����ձ�Ũ���ᡢ����ʳ��ˮMnO2+4HCl��Ũ��![]() MnCl2+2H2O+Cl2�����ն������������ֹ��Ⱦ����
MnCl2+2H2O+Cl2�����ն������������ֹ��Ⱦ����
��������
��1��������ƿ����ֱ�Ӽ��ȣ���˻�ȱ��ʯ���������������ж���Ҫβ����������ȱ���ձ�������ʢ������������Һ�����ɵ������к��лӷ������Ȼ����Լ�ˮ��������Ҫ�õ������������������Ҫ����ʳ��ˮ��Ũ���ᡣ
��2��ʵ���ҳ���Ũ����Ͷ������̼��ȷ�����Ӧ��ȡ��������Ӧ�Ļ�ѧ����ʽΪMnO2+4HCl��Ũ��![]() MnCl2+2H2O+Cl2����
MnCl2+2H2O+Cl2����
��3�������ж���Ҫβ���������������Լ����������Ƶ����������ն������������ֹ��Ⱦ������

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
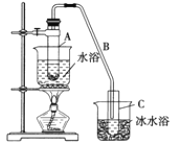
��Ȥ������ҵ���ϿƼ�������ϵ�д�����Ŀ��ij��ѧС���������������������װ��![]() ����ͼ
����ͼ![]() �Ի�����Ϊԭ���Ʊ�����ϩ��
�Ի�����Ϊԭ���Ʊ�����ϩ��
��֪��
�ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
������ | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 0.81 | ��103 | 83 | ������ˮ |
��1���Ʊ���Ʒ��12.5mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������________������B���˵�������е�������_________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����________________��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��____�㣨��ϡ����¡�������Һ����________�������ţ�ϴ�ӡ�
a.KMnO4��Һ b.ϡH2SO4 c.Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����__________________________��
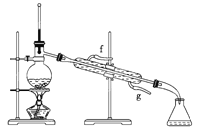
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��______���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����______��
a.����ʱ��70�濪ʼ�ռ���Ʒ
b.������ʵ����������
c.�Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_______��
a.�����Ը��������Һ b.�ý����� c.�ⶨ�е�

