��Ŀ����
��֪�����ڵ�����Ԫ��X��Y��Z��W��M��ԭ��������������X�ǿ����к�����
���Ԫ�أ��䵥�ʵĻ�ѧ���ʷdz��ȶ���Yԭ�ӵ������ֻ��2�����ӣ�Z���ʿ��Ƴɰ뵼����ϣ�WԪ���γɵĵ���Ϊ����ɫ�Ĺ��壬MԪ���γɵĵ���Ϊ����ɫ���壮��ش��������⣺
��1��XԪ�صķ����� ,�������ڱ���λ��Ϊ ��
��2��Z�������ᄃ������Ϊ ,��ѧʽ��: ��
��3��X��Y���γ����ӻ�����û�����ĵ���ʽΪ ��
��4��X������������ˮ������Y�������ﷴӦ�Ļ�ѧ����ʽΪ ��
��5��X��W�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ���ĽṹʽΪ ��
��6��W��M���ǽϻ��õķǽ���Ԫ�أ���ʵ����ʵ����������Ԫ�صķǽ�����ǿ�����÷���ʽ��д������˵���� ��
���Ԫ�أ��䵥�ʵĻ�ѧ���ʷdz��ȶ���Yԭ�ӵ������ֻ��2�����ӣ�Z���ʿ��Ƴɰ뵼����ϣ�WԪ���γɵĵ���Ϊ����ɫ�Ĺ��壬MԪ���γɵĵ���Ϊ����ɫ���壮��ش��������⣺
��1��XԪ�صķ����� ,�������ڱ���λ��Ϊ ��
��2��Z�������ᄃ������Ϊ ,��ѧʽ��: ��
��3��X��Y���γ����ӻ�����û�����ĵ���ʽΪ ��
��4��X������������ˮ������Y�������ﷴӦ�Ļ�ѧ����ʽΪ ��
��5��X��W�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ���ĽṹʽΪ ��
��6��W��M���ǽϻ��õķǽ���Ԫ�أ���ʵ����ʵ����������Ԫ�صķǽ�����ǿ�����÷���ʽ��д������˵���� ��
��1��N���ڶ����ڵڢ�A�壨2��ԭ�Ӿ��� ��SiO2
��3��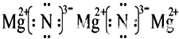
��4��2HNO3+MgO=Mg(NO3)2+2H2O
��5��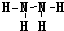
��6��Cl2+H2S=2HCl+S��
��3��
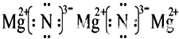
��4��2HNO3+MgO=Mg(NO3)2+2H2O
��5��
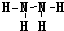
��6��Cl2+H2S=2HCl+S��
���������X�ǿ����к�������Ԫ�أ�XΪ��Ԫ�أ�Z���ʿ��Ƴɰ뵼����ϣ�ZΪ��Ԫ�أ���Y��þԪ�أ�WԪ���γɵĵ���Ϊ����ɫ�Ĺ��壬MԪ���γɵĵ���Ϊ����ɫ���壬��W��MΪ���ȡ�
��1��XԪ�ط�����N���ǵ������ڵڢ�A��Ԫ�أ�
��2��Z����������SiO2������ԭ�Ӿ��壻
��3��X��Y�γɵ����ӻ�����ΪMg3N2����ʽΪ
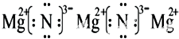
��4��X������������ˮ������HNO3��Y����������MgO�����߷�Ӧ�Ļ�ѧ����ʽΪ2HNO3+MgO=Mg(NO3)2+2H20��
��5��X��W�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ���ΪN2H4����ΪH2S, N2H4�ĽṹʽΪ
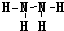 ��
����6��S�ķǽ����Ա�Cl����������Cl2��H2S��Ӧ�û�S��

��ϰ��ϵ�д�
�����Ŀ