��Ŀ����
��14�֣��к��ȵIJⶨʵ��Ĺؼ���Ҫ�Ƚ�ȷ������һ�������ʵ���Ũ�ȵ���Һ����ʵ�������Ҫ��������������ɢʧ��Ҫ��Ƚ�ȷ�ز�������Ӧǰ����Һ�¶ȵı仯���ش��������⣺
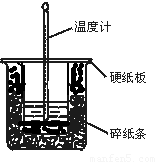
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2���ڴ�С�ձ�֮����������ĭ����ֽ������������____________________�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ��ƫС����Ӱ�족)
��3����һ���������к���ʵ�飬�¶ȼ���Ҫʹ��______ �Σ�
��4����ʵ�鳣��0��50 mol��L��1 HCl��0��55 mol��L��1��NaOH��Һ��50 mL��������HCl��NaOH��Һ���ܶȶ�����Ϊ1 g/cm3���кͺ����ɵ���Һ�ı�����C = 4.18 J/��g���棩����Ӧ���¶������ˡ�t, ������1molˮʱ�ķ�Ӧ�Ȧ�H=___________ kJ/mol�������ʽ����
��5�������50mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)���������� ��
��1�� ���β�������� ��2�����¡����ȣ�ƫС ��3��3 ��
��4����0.418��t/0.025 ��5������ȣ���Ϊ���������Ҫ����������
����������1���ڲⶨ�к��ȵ�ʵ����Ϊ��ʹ��Һ��Ͼ��У���ʵ���������Ҫ���裬����װ��ͼ��֪��ȱ�ٻ��β�����������
��2��Ϊ�˼�����������ʧ��Ҫ�ڴ�С�ձ�֮����������ĭ�����º��ȵ����á����û�и�Ӳ�ʰ壬������������ʧ�����²ⶨ���ƫС��
��3�����������������Һ���¶ȶ���Ҫ��������Ӧ����Ҫ�������Һ���¶ȣ���������ʹ��3�Ρ�
��4��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ������֮����100g�����Է�Ӧ�����зų���������100g��4.18J/(g•�棩����t�棽0.418��tkJ����Ϊ50mL0.50mol/L������50mL0.55mol/L
NaOH��Һ��Ӧ����ˮ��0.025mol�����ԡ�H����0.418��t/0.025kJ/mol��
��5����Ϊ����������û����Һ�д��ڵ���ƽ�⣬�����������ȵģ����Էų�������Ҫ�١�
