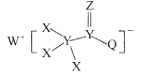
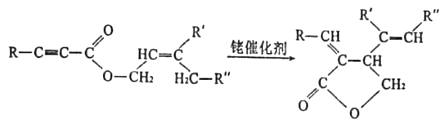
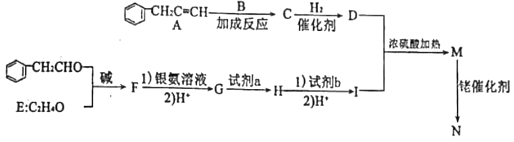
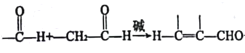
��Ŀ����
����Ŀ��������ѧ֪ʶ���ش��������⣺
(1)��������(Na2FeO4)����Ϊ��Ч�����ˮ����������Ħ��������__________���������ƿ���ˮ��Ӧ����Fe(OH)3���塢�������ƺ�������д����Ӧ�����ӷ���ʽ��_____________��
(2)��άͨ����ⷨ�����Ƶ��˽����ƣ����ʮ���ڣ���ҵ�ϲ������ۺ��������Ƹ������ڵķ����Ƶ���������ͬʱ���Fe3O4��������д���÷�Ӧ�Ļ�ѧ����ʽ_____________���÷�Ӧ�����ڵ���ʵ��������������������________________���ѧʽ�������Ƶ�22.4L(����Ϊ��״����)��������ת�Ƶ��ӵ���ĿΪ________________��
(3)Ư�۳�������ˮ����ˮ����й�P����Ⱦ�Ļ�������������Ҫ�ɷ����Ȼ��ƺʹ������[Ca(ClO)2]����Ч�ɷ�Ϊ������ơ�Ϊ���ijƯ�۵���Ч�ɷֺ�������ȡA gƯ����Ʒ�ܽ⣬��������Һ��ͨ��CO2�����ٲ�������Ϊֹ����Ӧ�Ļ�ѧ����ʽΪCa(ClO)2+CO2+H2O=CaCO3��+2HClO������Ӧ���ɴ�����(HClO)�����ʵ���ΪKmol�����Ư������Ч�ɷֵ���������Ϊ___________%���ú�A��K��ʽ�ӱ�ʾ����
���𰸡�166g/mol 4FeO42-+10H2O=4Fe(OH)3(����)+8OH-+3O2�� 3Fe+4NaOH=4Na��+Fe3O4+2H2�� NaOH 2NA��1.204��1024 ![]()
��������
(1)Ħ��������g/molΪ��λ����ֵ�ϵ�������Է������������ݵ���ת���غ㡢����غ㡢ԭ���غ���ƽ��д���ӷ���ʽ��
(2)�������֪��Fe��NaOH�ڸ�������������Na������Fe3O4��H2����ƽ��д��ѧ����ʽ����Ӧ�����ڵ���ʵ��������������������NaOH����Ӧ��Na��HԪ�ػ��ϼ۽��ͣ����ݷ���ʽ����������������������NaԪ�ء�HԪ�ػ��ϼ۱仯����ת�Ƶ�����Ŀ��
(3)����HClO��������Ca(ClO)2���������ٸ������������Ķ���ʽ���㡣
(1)Na2FeO4����Է�������Ϊ166������Ħ������Ϊ166g/mol���������ƿ���ˮ��Ӧ����Fe(OH)3���塢�������ƺ���������Ӧ�����ӷ���ʽ��4FeO42-+10H2O=4Fe(OH)3(����)+8OH-+3O2����
(2)�������֪��Fe��NaOH�ڸ�������������Na������Fe3O4��H2����Ӧ����ʽΪ��3Fe+4NaOH![]() 4Na��+Fe3O4+2H2������Ӧ�����ڵ���ʵ��������������������NaOH��Na�����ʵ���n(Na)=224L��22.4L/mol=1mol���ɷ���ʽ��֪��������Ϊ1mol��
4Na��+Fe3O4+2H2������Ӧ�����ڵ���ʵ��������������������NaOH��Na�����ʵ���n(Na)=224L��22.4L/mol=1mol���ɷ���ʽ��֪��������Ϊ1mol��![]() =0.5mol����ת�Ƶ��ӵ����ʵ���n(e-)=1mol��1+0.5mol��2=2mol����ת�Ƶ�����ĿN(e-)=2mol��NA/mol=2mol��(6.02��1023mol-1)=1.204��1024��
=0.5mol����ת�Ƶ��ӵ����ʵ���n(e-)=1mol��1+0.5mol��2=2mol����ת�Ƶ�����ĿN(e-)=2mol��NA/mol=2mol��(6.02��1023mol-1)=1.204��1024��
(3)��Ca(ClO)2+CO2+H2O=CaCO3��+2HClO��֪��n[Ca(ClO)2]=![]() n(HClO)=
n(HClO)=![]() ��Kmol=0.5Kmol�����Ư������Ч�ɷֵ���������
��Kmol=0.5Kmol�����Ư������Ч�ɷֵ���������![]() ��100%=
��100%=![]() %��
%��

 �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
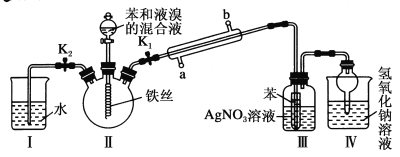
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�����Ŀ������ͼ��ʾװ�ý���ʵ�飬���ж�ʵ������Ľ��Ͳ���������

ѡ�� | ���Լ� | �������� | ���� |
A | Ʒ����Һ | ��Һ��ɫ | SO2����Ư���� |
B | Na2SiO3��Һ | ������״���� | ���ԣ� H2SO3 ��H2SiO3 |
C | ����KMnO4��Һ | ��ɫ��ȥ | SO2���л�ԭ�� |
D | Ba(NO3)2��Һ | ���ɰ�ɫ���� | SO32����Ba2������ ��ɫBaSO3���� |
A.AB.BC.CD.D
����Ŀ�������ܱ��ʼ�����һ�ֿ����ӳ����ڵ��Լ��������500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
�ɷ� | ����(g) | Ħ������(gmol1) |
���� | 25.00 | 342 |
����� | 0.87 | 174 |
��˾ƥ�� | 0.17 | 180 |
������� | 0.316 | 158 |
������ | 0.0075 | 170 |
(1)���������ܱ��ʼ����ijɷ��У����ڷǵ���ʵ���______������ɱ��������____
A. �������B. �����C. ����D. ������E. ˮ
(2) ����1L�������ʻ����ʼ�����Ҫ�������______mol��
(3)����Һ���ƹ����У����в�����ʹ���ƽ����Ӱ�����______��
A.����ʱ��������ƿ�̶���
B.����ƿ��ʹ��ǰδ�����������������ˮ
C.����ƿ��ʹ��ǰ��������һ�����ʵ���Ũ�ȵ��Ȼ�����Һδϴ��
D.����ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ���δ���κδ���
(4)���ʻ����ʼ�����K+(��˾ƥ���в���K+)�����ʵ���Ũ��Ϊ______mol/L��
(5)����ø�����أ�K2FeO4������������Ч������ã�ʪ���Ʊ�������صķ�Ӧ��ϵ������������Fe(OH)3��ClO-��OH-��FeO42-��Cl-��H2O.���������£��������뻹ԭ�������ʵ����ı�Ϊ3:2������Ӧ��д������ƽʪ���Ʊ�������ص����ӷ�Ӧ����ʽ___________________________________________________________