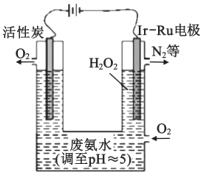
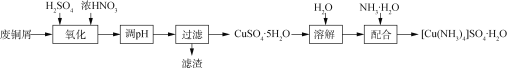
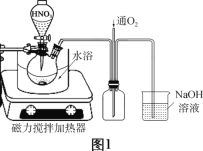
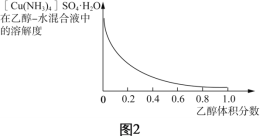
��Ŀ����
����Ŀ��1906�꣬������60����¡�200������ѹ�������£����(Os)���������״γɹ��õ��˰��������ʽϵ͡����ſ�ѧ�Ľ����Լ���ѧ���ǶԴ������о��Ľ������ڹ�ҵ���ձ��������ý����Ҫ�ɷ�ΪFe3O4����������K2O��Al2O3��CaO��MgO��CoO�ȣ����ϳɰ��Ĵ������������˺ϳɰ��IJ��ʡ��ش��������⣺
��1����֪Ԫ��Co(�ܣ���ԭ�Ӻ�����27�����ӣ����Ԫ�ػ�̬ԭ�Ӽ۵����Ų�ʽΪ___��
��2��Ԫ��Fe�Ļ�̬ԭ�Ӻ���δ�ɶԵ�����Ϊ___��Fe2+��Fe3+��Ƚϣ�___���ȶ���
��3��C��N��O����ͬһ���ڣ����е�һ����������___���縺��������___�����������γɵļ��⻯��е�Ӹߵ��͵�˳��Ϊ___��
��4��NH3������Nԭ�ӵ��ӻ���ʽΪ___��������������ˮ���ܽ��1��700)�����˰�������ˮ������Ӧ�⣬��������ԭ��ֱ���___��___��
��5���ҹ�����ϵ�����ػ��ʹ�õ�Һ̬ȼ����Ҫ��ƫ�����£۽ṹ��ʽ��(CH3)2NNH2���ɿ�������(NH2NH2)��ͬһ��ԭ���ϵ�������ԭ�ӱ���ȡ���ݺ�������������ȼ��ʱ������Ӧ��(CH3)2NNH2+2N2O4=2CO2+4H2O+3N2�����÷�Ӧ����1molN2O4ʱ���γ�___mol������
���𰸡�3d74s2 4 Fe3+ N O H2O��NH3��CH4 sp3 ������ˮ��Ϊ���Է��ӻ��������� ����������ˮ����֮������γ���� 5
��������
(1)��֪Ԫ��Co(��)��ԭ�Ӻ�����27�����ӣ�����������ԭ�Ӻ�������������Ԫ�ػ�̬ԭ�Ӻ�������Ų�ʽΪ��[Ar]3d74s2���۵����Ų�ʽΪ3d74s2��
(2)Ԫ��Fe�Ļ�̬ԭ�Ӻ����Ų�ʽΪ1s22s22p63s23p63d64s2��3d�������6�����ӣ����ݺ��ع���������ռ��һ���չ���������δ�ɶԵ�����Ϊ4��Fe2+�۲�����Ų�Ϊ1s22s22p63s23p63d6��Fe3+�۲�����Ų�Ϊ1s22s22p63s23p63d5��Fe3+��3d����ϴ��ڰ�����ȶ�״̬��Fe3+���ȶ���
(3)C��N��O����ͬһ���ڣ���˵��������һ������������Nԭ�Ӻ������2p����ϵ��Ӵ��ڰ�����ȶ�״̬����һ���������Ԫ�ش����е�һ����������ΪNԪ�أ�ͬһ���ڣ���˵�������縺�������縺��������O��C��N��O����Ԫ�ص�����⻯��ֱ�Ϊ��CH4��NH3��H2O�����ڰ�����ˮ�����γ�����������߷е�Ӹߵ��͵�˳��Ϊ��H2O��NH3��CH4��
(4)NH3������Nԭ�ӵļ۵��Ӷ���=3+![]() =4����N�ӻ���ʽΪsp3��������������ˮ(�ܽ��1��700)�����˰�������ˮ������Ӧ�⣬��������ԭ��ֱ��ǰ�����ˮ��Ϊ���Է��ӻ��������ܡ�����������ˮ����֮������γ������
=4����N�ӻ���ʽΪsp3��������������ˮ(�ܽ��1��700)�����˰�������ˮ������Ӧ�⣬��������ԭ��ֱ��ǰ�����ˮ��Ϊ���Է��ӻ��������ܡ�����������ˮ����֮������γ������
(5)һ��N2�����ɵ���������϶��ɣ������к���һ��������2��������һ��CO2�����к�������̼��˫����ÿ��˫����һ��������1�����������ݷ�Ӧ(CH3)2NNH2+2N2O4=2CO2+4H2O+3N2�����÷�Ӧ����1molN2O4ʱ������1mol CO2��![]() mol N2�����γ����������ʵ���=1mol��2+
mol N2�����γ����������ʵ���=1mol��2+![]() mol��2=5mol��
mol��2=5mol��

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�