��Ŀ����
��16�֣������û�ѧ��Ӧԭ�������֪ʶ�о�̼���仯��������ʡ�
��1���ҹ���������̼���о���ȡ���ش��չ���õ绡���ϳɵ�̼�����г����д���̼
�����������ʣ�������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ��
___C + ___K2Cr2O7 + _______=" ___" CO2�� + ___K2SO4 + ___Cr2(SO4)3 +___H2O
����ɲ���ƽ������ѧ����ʽ��
��2����ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��
CO(g)+ 2H2(g) CH3OH(g) ��H1����116 kJ��mol-1
CH3OH(g) ��H1����116 kJ��mol-1
�����д�ʩ������������÷�Ӧ�ķ�Ӧ���ʵ���____________��
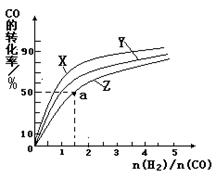
���ں��������кϳɼ״������¶ȷֱ�Ϊ230�桢250���270��ʱ��CO��ת������n(H2)/n(CO)����ʼ��ɱȵĹ�ϵ����ͼ��ʾ����֪�������1L����ʼʱCO�����ʵ�����Ϊ1mol���ݴ��ж������������¶��У�����Z��Ӧ���¶���__________������ͼ��a���Ӧ�����ݣ�����÷�Ӧ�ڶ�Ӧ�¶��µ�ƽ�ⳣ��K =_______________________��
����֪��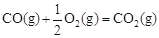 ��H2����283 kJ��mol-1
��H2����283 kJ��mol-1
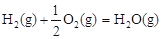 ��H3����242 kJ��mol-1
��H3����242 kJ��mol-1
���ʾ1mol��̬�״���ȫȼ������CO 2��ˮ�������Ȼ�ѧ����ʽΪ_________��
��3��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ����ܶȻ�����Ksp=
c(Ca2+)��c(CO32��)=2.8��10?9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ5.6��10 -5 mol/L �������ɳ�������CaCl2��Һ����СŨ��Ϊ________________________��
��1���ҹ���������̼���о���ȡ���ش��չ���õ绡���ϳɵ�̼�����г����д���̼
�����������ʣ�������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ��
___C + ___K2Cr2O7 + _______=" ___" CO2�� + ___K2SO4 + ___Cr2(SO4)3 +___H2O
����ɲ���ƽ������ѧ����ʽ��
��2����ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��
CO(g)+ 2H2(g)
 CH3OH(g) ��H1����116 kJ��mol-1
CH3OH(g) ��H1����116 kJ��mol-1�����д�ʩ������������÷�Ӧ�ķ�Ӧ���ʵ���____________��
| A��ʹ�ø�Ч���� | B�����ͷ�Ӧ�¶� |
| C��������ϵѹǿ | D�����Ͻ�CH3OH�ӷ�Ӧ������з������ |

����֪��
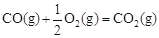 ��H2����283 kJ��mol-1
��H2����283 kJ��mol-1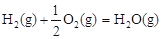 ��H3����242 kJ��mol-1
��H3����242 kJ��mol-1���ʾ1mol��̬�״���ȫȼ������CO 2��ˮ�������Ȼ�ѧ����ʽΪ_________��
��3��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ����ܶȻ�����Ksp=
c(Ca2+)��c(CO32��)=2.8��10?9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ5.6��10 -5 mol/L �������ɳ�������CaCl2��Һ����СŨ��Ϊ________________________��
��1��3C + 2K2Cr2O7 +8H2SO4 = 3CO2�� + 2K2SO4 + 2Cr2(SO4)3 +8H2O��3�֣�����H2SO4��1�֣�
��2����AC��2�֣���1�֣���ѡ���ѡ1������1�֣�����Ϊֹ�����������֣�
��270�棨2�֣���λ��1�֣� 4L2/mol2��3�֣���д��λҲ���֣�
��CH3OH(g)+3/2O2(g) CO2(g)+2H2O(g) ��H����651kJ��mol-1��3�֣�
CO2(g)+2H2O(g) ��H����651kJ��mol-1��3�֣�
��3��2��10-4mol/L��3�֣���д��λ��1�֣�
��2����AC��2�֣���1�֣���ѡ���ѡ1������1�֣�����Ϊֹ�����������֣�
��270�棨2�֣���λ��1�֣� 4L2/mol2��3�֣���д��λҲ���֣�
��CH3OH(g)+3/2O2(g)
 CO2(g)+2H2O(g) ��H����651kJ��mol-1��3�֣�
CO2(g)+2H2O(g) ��H����651kJ��mol-1��3�֣���3��2��10-4mol/L��3�֣���д��λ��1�֣�
��1������������ԭ��Ӧ����ʽ����ƽ����ƽ�������ǵ��ӵĵ�ʧ�غ㡣̼�ǻ�ԭ�������ϼ�����4����λ��K2Cr2O7������������Ԫ�صĻ��ϼ۽���3����λ������1mol�������õ�6mol���ӣ����������ͻ�ԭ�������ʵ���֮����2�U3�����Է���ʽΪ3C + 2K2Cr2O7 +8H2SO4 = 3CO2�� + 2K2SO4 + 2Cr2(SO4)3 +8H2O��
��2���ٿ�����������Է�Ӧ���ʵ�Ӱ�졣����ѹǿ��ʹ�ô�����������Ӧ���ʣ�AC��ȷ��BD�ǽ��ͷ�Ӧ���ʡ�
�������Ƿ��ȷ�Ӧ�������¶�Խ�ߣ�CO��ת����Խ�͡����Z��ʾ����270�档a���ת������50����CO����ʼŨ����1������������1.5�����ĵ�COŨ����0.5��������1�����ɼ״���0.5������ƽ�ⳣ����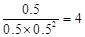 ��
��
�ۿ����˹���ɵ�Ӧ�á����ݷ�Ӧ��CO(g)+ 2H2(g) CH3OH(g)����
CH3OH(g)����
��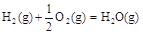 ��֪���ڣ��٣��ۡ�2�ɵ�H3OH(g)+3/2O2(g)
��֪���ڣ��٣��ۡ�2�ɵ�H3OH(g)+3/2O2(g) CO2(g)+2H2O(g)�����Է�Ӧ���ǣ�283 kJ��mol-1��116 kJ��mol-1��242 kJ��mol-1��2����651kJ��mol-1��
CO2(g)+2H2O(g)�����Է�Ӧ���ǣ�283 kJ��mol-1��116 kJ��mol-1��242 kJ��mol-1��2����651kJ��mol-1��
��3������Ksp= c(Ca2+)��c(CO32��)=2.8��10?9��֪c(Ca2+)��Ksp/c(CO32��)��2.8��10?9/2.8��10 -5��1��10-4mol/L����������CaCl2��Һ����СŨ��Ϊ1��10-4mol/L��2��2��10-4mol/L
��2���ٿ�����������Է�Ӧ���ʵ�Ӱ�졣����ѹǿ��ʹ�ô�����������Ӧ���ʣ�AC��ȷ��BD�ǽ��ͷ�Ӧ���ʡ�
�������Ƿ��ȷ�Ӧ�������¶�Խ�ߣ�CO��ת����Խ�͡����Z��ʾ����270�档a���ת������50����CO����ʼŨ����1������������1.5�����ĵ�COŨ����0.5��������1�����ɼ״���0.5������ƽ�ⳣ����
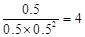 ��
���ۿ����˹���ɵ�Ӧ�á����ݷ�Ӧ��CO(g)+ 2H2(g)
 CH3OH(g)����
CH3OH(g)����
��
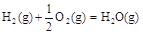 ��֪���ڣ��٣��ۡ�2�ɵ�H3OH(g)+3/2O2(g)
��֪���ڣ��٣��ۡ�2�ɵ�H3OH(g)+3/2O2(g) CO2(g)+2H2O(g)�����Է�Ӧ���ǣ�283 kJ��mol-1��116 kJ��mol-1��242 kJ��mol-1��2����651kJ��mol-1��
CO2(g)+2H2O(g)�����Է�Ӧ���ǣ�283 kJ��mol-1��116 kJ��mol-1��242 kJ��mol-1��2����651kJ��mol-1����3������Ksp= c(Ca2+)��c(CO32��)=2.8��10?9��֪c(Ca2+)��Ksp/c(CO32��)��2.8��10?9/2.8��10 -5��1��10-4mol/L����������CaCl2��Һ����СŨ��Ϊ1��10-4mol/L��2��2��10-4mol/L

��ϰ��ϵ�д�
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
�����Ŀ
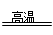 Si+2CO���������������뻹ԭ�������ʵ���֮��Ϊ( )
Si+2CO���������������뻹ԭ�������ʵ���֮��Ϊ( )