��Ŀ����
CuSO4��Һ����ѧ��ѧ����ũҵ�����г�����һ���Լ���
(1)ijͬѧ����CuSO4��Һʱ����ʢ��һ��������ͭ������ձ��м�������������ˮ�������Ͻ��裬����õ�����Һ������Ϊ�ǹ���û����ȫ�ܽ⣬���Ƕ�����Һ���ȣ�������ֻ��Ǹ������ˣ�ԭ����_______________����������ձ��м�����һ������____________��Һ���õ��˳����CuSO4��Һ��
(2)��ͬѧ�����Ƶõ�CuSO4��Һ������ͼ��������ʵ��̽����
(1)ijͬѧ����CuSO4��Һʱ����ʢ��һ��������ͭ������ձ��м�������������ˮ�������Ͻ��裬����õ�����Һ������Ϊ�ǹ���û����ȫ�ܽ⣬���Ƕ�����Һ���ȣ�������ֻ��Ǹ������ˣ�ԭ����_______________����������ձ��м�����һ������____________��Һ���õ��˳����CuSO4��Һ��
(2)��ͬѧ�����Ƶõ�CuSO4��Һ������ͼ��������ʵ��̽����

��ͼһ�Ǹ��ݷ�ӦZn+CuSO4=Cu+ZnSO4��Ƴɵ�пͭԭ��ء��������Һ����____(�ZnSO4����
��CuSO4��)��Һ��Cu���ĵ缫��Ӧʽ��_____________
��ͼ���У�I�Ǽ���ȼ�ϵ��(�������ҺΪKOH��Һ���Ľṹʾ��ͼ����ͬѧ���ڢ���ʵ�����϶�ͭ����b��ͨ�����____(�CH4����O2��)��a���缫�Ϸ����ĵ缫��Ӧ��____________��
(3)������(CuSO4��5H2O)����ʯ�Һ�ˮ����ͬ������ϣ����ɵõ���ɲ�ͬ�IJ�����Һ��ɱ������������Ч�ɷ�Ϊ���ܵļ�ʽ����ͭ[���ɱ�ʾΪxCuSO4��yCu(OH)2]��Ϊ�ⶨij��ʽ����ͭ�������ᾧˮ������ɽ���������ʵ�飺ȡ�������ļ�ʽ����ͭ��Ʒ���ݣ�һ�ݵμ�ϡ������ǡ����ȫ�ܽ⣬��һ�ݸ������� ��ֻ�õ�CuO���塣����������ʾn(HCl)��n(CuO)=3��2����ü�ʽ����ͭ�Ļ�ѧʽ��x��y=____��
��CuSO4��)��Һ��Cu���ĵ缫��Ӧʽ��_____________
��ͼ���У�I�Ǽ���ȼ�ϵ��(�������ҺΪKOH��Һ���Ľṹʾ��ͼ����ͬѧ���ڢ���ʵ�����϶�ͭ����b��ͨ�����____(�CH4����O2��)��a���缫�Ϸ����ĵ缫��Ӧ��____________��
(3)������(CuSO4��5H2O)����ʯ�Һ�ˮ����ͬ������ϣ����ɵõ���ɲ�ͬ�IJ�����Һ��ɱ������������Ч�ɷ�Ϊ���ܵļ�ʽ����ͭ[���ɱ�ʾΪxCuSO4��yCu(OH)2]��Ϊ�ⶨij��ʽ����ͭ�������ᾧˮ������ɽ���������ʵ�飺ȡ�������ļ�ʽ����ͭ��Ʒ���ݣ�һ�ݵμ�ϡ������ǡ����ȫ�ܽ⣬��һ�ݸ������� ��ֻ�õ�CuO���塣����������ʾn(HCl)��n(CuO)=3��2����ü�ʽ����ͭ�Ļ�ѧʽ��x��y=____��
(1)Cu2++2H2O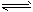 Cu(OH)2+2H+ ��H>0�����ȴٽ���Cu2+ˮ�⣬�����˸����������Cu(OH)2��H2SO4
Cu(OH)2+2H+ ��H>0�����ȴٽ���Cu2+ˮ�⣬�����˸����������Cu(OH)2��H2SO4
(2)��ZnSO4��Cu2++2e-=Cu����O2��CH4-8e-+10OH-=CO32-+7H2O
(3)1��3
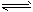 Cu(OH)2+2H+ ��H>0�����ȴٽ���Cu2+ˮ�⣬�����˸����������Cu(OH)2��H2SO4
Cu(OH)2+2H+ ��H>0�����ȴٽ���Cu2+ˮ�⣬�����˸����������Cu(OH)2��H2SO4 (2)��ZnSO4��Cu2++2e-=Cu����O2��CH4-8e-+10OH-=CO32-+7H2O
(3)1��3

��ϰ��ϵ�д�
�����Ŀ

 Cu��OH��2+2H+
Cu��OH��2+2H+


 ������
������