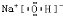��Ŀ����
X��Y��Z�����ֶ�����Ԫ�أ��ס��ҡ���Ϊ�������ת����ϵ���£�
��ش�
(1)��ʵ����ͼ��ת������X��Y��Z�������______���ǽ���Ԫ�ء�
(2)���ס��ҷ����о�����10�����ӣ��Ҽ����к���3��ԭ�ӣ��ҷ����к���4��ԭ�ӣ�������_________������_________��
(3)���͵���Z��Ϊ����ɫ���壬���ˮ��Ӧ�Ļ�ѧ����ʽΪ______________����֪
(1)1
(2)NH3 NO
(3)2Na2O2+2H2O![]() 4NaOH+O2��
4NaOH+O2��
S(s)+O2(g)![]() SO2(g)����H=
SO2(g)����H=
������(1)�ڼס��ҡ����У�X��Y��Z����Ԫ������������Ԫ�����Ը����ϼۣ����������һ�ֽ���Ԫ��.
(2)����10���ӵķ�����CH4��NH3��H2O��HF�����������У��ô�.
(3)��ΪNa2O2��ZΪS.

��ϰ��ϵ�д�
�����Ŀ