��Ŀ����
������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ����1�dz����¼�������ĵ���ƽ�ⳣ����Ka��������ĵ���ƽ�ⳣ����Kb������2�dz����¼����ѣ���������ܶȻ�������Ksp������1
| ���� | ����ƽ�ⳣ����Ka��Kb�� |
| CH3COOH | 1.8×10-5 |
| HNO2 | 4.6×10-4 |
| HCN | 5×1010 |
| HClO | 3×10-8 |
| NH2?H2O | 1.8×10-5 |
| �ѣ������� | ��Ȼ�������Ksp�� |
| BaSO4 | 1×10-10 |
| BaCO3 | 2.6×10-9 |
| CaSO4 | 7×10-5 |
| CaCO3 | 5×10-9 |
��1��CH3COONH4��ˮ��Һ��______��ѡ����ԡ��������ԡ����ԡ�����������______����Һ�и�����Ũ�ȴ�С��ϵ��______��
��2�����ʵ���֮��Ϊ1��1��NaCN��HCN�Ļ����Һ����pH��7������Һ������Ũ�ȴӴ�С������Ϊ______��
��3�����ʵ���Ũ�Ⱥ��������ͬ��NaNO2��NaCN������Һ����֪ǰ����Һ��������ĿΪn1��������Һ��������ĿΪn2�����й�ϵ��ȷ�ǣ�______��
A�� n1=n2 B�� n1��n2 C�� n1��n2 D��c��NO2-����c��CN-��
��2�����ʵ���֮��Ϊ1��1��NaCN��HCN�Ļ����Һ����pH��7��˵����������ӵ�ˮ��̶ȴ���������ĵ���̶ȣ�������Һ�ʼ��ԣ����ݵ���غ�֪�������Ӻ����������Ũ�ȵĹ�ϵ��
��3��������ĵ���ƽ�ⳣ�����������ᣬ�������������ˮ��̶ȴ��������������ˮ��̶ȣ�ˮ��̶�Խ��������ĿԽ�٣�
����⣺��1��ͨ�����Ϸ���֪��笠����Ӻʹ�������ӵ�ˮ��̶���ȣ�������Һ��C��OH-��=C��H+������Һ�����ԣ����ݵ�����֪����Һ��C��CH3COO-��=C��Na+������Һ��ˮ�ĵ��������ģ�����C��CH3COO-����C��OH-������������Һ�и�������Ũ�ȴ�С˳����C��CH3COO-��=C��Na+����C��OH-��=C��H+����
�ʴ�Ϊ�����ԣ����������ˮ���ݱ�1�еĵ���ƽ�ⳣ�����������������Ӻ�笠����ӽ����������������������ʵij̶�һ����������Һ��C��OH-��=C��H+����C��CH3COO-��=C��Na+����C��OH-��=C��H+����
��2�����ʵ���֮��Ϊ1��1��NaCN��HCN�Ļ����Һ����pH��7��˵����������ӵ�ˮ��̶ȴ���������ĵ���̶ȣ�������ҺC��OH-����C��H+�����ʼ��ԣ����ݵ���غ�֪��C��OH-��+c��CN-��=C��H+��+C��Na+��������c��CN-����C��Na+�������Ը�����Ũ�ȴ�С˳����C��Na+����c��CN-����C��OH-����C��H+����
�ʴ�Ϊ��C��Na+����c��CN-����C��OH-����C��H+����
��3��������ĵ���ƽ�ⳣ�����������ᣬ�������������ˮ��̶ȴ��������������ˮ��̶ȣ�ˮ��̶�Խ��������ĿԽ�٣���������������Һ�е�������Ŀ�����軯���е�������Ŀ����ѡB��
���������⿼����������ʵĵ���ƽ�⣬��ȷ����ƽ�ⳣ���ĺ����ǽⱾ��Ĺؼ�����ϵ���غ���������ɣ��Ѷ��еȣ�

��12�֣�I������������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±�1�dz����¼�������ĵ���ƽ�ⳣ����Ka��������ĵ���ƽ�ⳣ����Kb������1
|
���� |
����ƽ�ⳣ����Ka�� Kb�� |
|
CH3COOH |
1��8��10-5 |
|
HNO2 |
4��6��10-4 |
|
HCN |
5��10-10 |
|
HClO |
3��10-8 |
|
NH3��H2O |
1��8��10-5 |
��ش��������⣺
�����������У������������� ���û�ѧʽ��ʾ����������ʹ������Һ��CH3COOH�ĵ���̶���������ƽ�ⳣ������IJ����� ������ţ���
A�������¶�
B����ˮϡ��
C����������CH3COONa����
D��������������
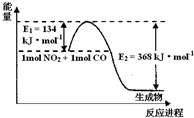
II�����ǵ����Ϻ�����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش��������⣺��ͼ���漰����Ϊ��̬��
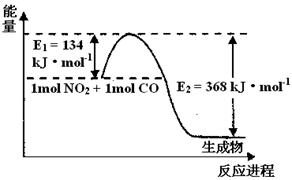
��1����ͼ��1 mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��
��2����0��5L���ܱ������У�һ�����ĵ����������������»�ѧ��Ӧ��
N2��g��+3H2��g��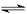 2NH3��g����H<0���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���������������⡣
2NH3��g����H<0���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���������������⡣
|
t/�� |
200 |
300 |
400 |
|
K |
K1 |
K2 |
0��5 |
���ԱȽ�K1��K2�Ĵ�С��K1_ K2����д��>������=����<������
�����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������____���������ĸ����
a��������N2��H2��NH3��Ũ��֮��Ϊ1��3��2 b��v��N2����=3v��H2����
c��������ѹǿ���ֲ��� d�����������ܶȱ��ֲ���
����400��ʱ�� �����NH3��N2��H2�����ʵ����ֱ�Ϊ1mol��2mol��3molʱ����÷�Ӧ��v��N2����_ _ v��N2��������д��>������=����<����
����������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±��dz����¼���������ʵĵ���ƽ�ⳣ����
|
���� |
���볣��(Ka��Kb) |
|
CH3COOH |
1��8��10��5 |
|
HNO2 |
4��6��10��4 |
|
HCN |
5��10��10 |
|
HClO |
3��10��8 |
|
NH3��H2O |
1��8��10��5 |
��ش��������⣺
(1)�����������У������������ǣߣߣߣߣߣߣ� (�û�ѧʽ��ʾ)��������ʹ����
��Һ��CH3COOH�ĵ���̶��������볣������IJ����ǣߣߣߣߣ� (�����)��
A�������¶�
B����ˮϡ��
C����������CH3COONa����
D��������������
(2)CH3COONH4��ˮ��Һ�ʣߣߣߣߣ� (ѡ����ԡ������ԡ����ԡ�)�������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���Һ�и�����Ũ�ȴ�С�Ĺ�ϵ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(3)���ʵ���1��1��NaCN��HCN�Ļ����Һ����pH��7������Һ�����ӵ�Ũ�ȴӴ�
��С����Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(4)��֪һЩ��������ܶȻ��������±���
|
���� |
FeS |
MnS |
Cus |
PbS |
HgS |
ZnS |
|
Ksp |
6.3��10-18 |
2.5��10-13 |
1.3��10-36 |
3.4��10-28 |
6.4��10-55 |
1.6��10-24 |
ij��ҵ��ˮ�к���Cu2����Pb2����Hg2������������˹�ҵ��ˮ�м�������� ��ȥ���ǡ�(ѡ�����)
��NaOH����FeS����Na2S
 I������������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±�1�dz����¼�������ĵ���ƽ�ⳣ����Ka��������ĵ���ƽ�ⳣ����Kb����
I������������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±�1�dz����¼�������ĵ���ƽ�ⳣ����Ka��������ĵ���ƽ�ⳣ����Kb����