��Ŀ����
��������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±��dz����¼�������ĵ���ƽ�ⳣ����Ka��������ĵ���ƽ�ⳣ����Kb������ش��������⣺
��1��CH3COONH4��ˮ��Һ��
���¶�t��ʱ��ijNaOHϡ��Һ��c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=12����ش���������
��2�����¶���ˮ�����ӻ�����KW=
��3����NaOH��Һ�����ʵ���Ũ����
��4������NaOH��Һ���ȣ�pH=
��5�����¶�����a=8��Ҫ�к�NaOH��Һ500mL����pH=2��������Һ�����Ϊ
| ���� | ����ƽ�ⳣ����Ka��Kb�� |
| CH3COOH | 1.8��10-3 |
| HNO3 | 4.6��10-4 |
| HCN | 5��10-10 |
| HClO | 3��10-8 |
| NH3?H2O | 1.8��10-5 |
����
����
��ѡ����ԡ��������ԡ����ԡ��������������������ˮ���ݱ�1�еĵ���ƽ�ⳣ�����������������Ӻ�笠����ӽ����������������������ʵij̶�һ����������Һ��C��OH-��=C��H+��
���������ˮ���ݱ�1�еĵ���ƽ�ⳣ�����������������Ӻ�笠����ӽ����������������������ʵij̶�һ����������Һ��C��OH-��=C��H+��
����Һ�и�����Ũ�ȴ�С��ϵ��C��CH3COO-��=C��Na+����C��OH-��=C��H+��
C��CH3COO-��=C��Na+����C��OH-��=C��H+��
�����¶�t��ʱ��ijNaOHϡ��Һ��c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=12����ش���������
��2�����¶���ˮ�����ӻ�����KW=
12
12
����3����NaOH��Һ�����ʵ���Ũ����
10-b
10-b
mol/L����NaOH��Һ����ˮ�������c��OH-����10-a
10-a
mol/L����4������NaOH��Һ���ȣ�pH=
��С
��С
��������С�䣩��5�����¶�����a=8��Ҫ�к�NaOH��Һ500mL����pH=2��������Һ�����Ϊ
5mL
5mL
��������I����1�������ᡢ��ĵ���ƽ�ⳣ��֪����ͬ�¶��£�����Ͱ�ˮ�ĵ���ƽ�ⳣ����ȣ��������̶���ȣ�笠����Ӻʹ�������ӵ�ˮ��̶���ȣ�������Һ������������Ũ�Ⱥ�������Ũ����ȣ���Һ�����ԣ��������Һ�У��εĵ���Ϊ����ˮ�ĵ���Ϊ�Σ�
II����2����Һ�е����ӻ�Kw=C��H+����c��OH-��=10-a��10-b=10-��a+b��=10-12��
��3����NaOH��Һ��NaOH�����ʵ���Ũ��ΪC��NaOH��=c��OH-��=10-bmol/L��������Һ�е����ӻ�Kw=C��H+����c��OH-��=10-12����NaOH��Һ����ˮ�������c��OH-��Ϊ10-a��
��4������NaOH��Һ���ȣ��ٽ�ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�����������Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ������������ҺPH��С��
��5��������ͼ�����ʵ�����ȼ��㣮
II����2����Һ�е����ӻ�Kw=C��H+����c��OH-��=10-a��10-b=10-��a+b��=10-12��
��3����NaOH��Һ��NaOH�����ʵ���Ũ��ΪC��NaOH��=c��OH-��=10-bmol/L��������Һ�е����ӻ�Kw=C��H+����c��OH-��=10-12����NaOH��Һ����ˮ�������c��OH-��Ϊ10-a��
��4������NaOH��Һ���ȣ��ٽ�ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�����������Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ������������ҺPH��С��
��5��������ͼ�����ʵ�����ȼ��㣮
����⣺��1��ͨ�����Ϸ���֪��笠����Ӻʹ�������ӵ�ˮ��̶���ȣ�������Һ��C��OH-��=C��H+������Һ�����ԣ����ݵ�����֪����Һ��C��CH3COO-��=C��Na+������Һ��ˮ�ĵ��������ģ�����C��CH3COO-����C��OH-������������Һ�и�������Ũ�ȴ�С˳����C��CH3COO-��=C��Na+����C��OH-��=C��H+����
�ʴ�Ϊ�����ԣ����������ˮ���ݱ�1�еĵ���ƽ�ⳣ�����������������Ӻ�笠����ӽ����������������������ʵij̶�һ����������Һ��C��OH-��=C��H+����C��CH3COO-��=C��Na+����C��OH-��=C��H+����
II����2����Һ�е����ӻ�Kw=C��H+����c��OH-��=10-a��10-b=10-��a+b��=10-12���ʴ�Ϊ��10-12��
��3����NaOH��Һ��NaOH�����ʵ���Ũ��ΪC��NaOH��=c��OH-��=10-bmol/L��������Һ�е����ӻ�Kw=C��H+����c��OH-��=10-12����NaOH��Һ����ˮ�������c��OH-��Ϊ10-a��
�ʴ�Ϊ��10-b mol/L��10-amol/L��
��4������NaOH��Һ���ȣ��ٽ�ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�����������Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ������������ҺPH��С��
�ʴ�Ϊ����С��
��5����ȫ�к�ʱ����ͼ�����ʵ�����ȣ�a=8�����������Ƶ����ʵ���Ũ��Ϊ0.0001mol/L���к�NaOH��Һ500mL����pH=2��������Һ���=
=5mL��
�ʴ�Ϊ��5mL��
�ʴ�Ϊ�����ԣ����������ˮ���ݱ�1�еĵ���ƽ�ⳣ�����������������Ӻ�笠����ӽ����������������������ʵij̶�һ����������Һ��C��OH-��=C��H+����C��CH3COO-��=C��Na+����C��OH-��=C��H+����
II����2����Һ�е����ӻ�Kw=C��H+����c��OH-��=10-a��10-b=10-��a+b��=10-12���ʴ�Ϊ��10-12��
��3����NaOH��Һ��NaOH�����ʵ���Ũ��ΪC��NaOH��=c��OH-��=10-bmol/L��������Һ�е����ӻ�Kw=C��H+����c��OH-��=10-12����NaOH��Һ����ˮ�������c��OH-��Ϊ10-a��
�ʴ�Ϊ��10-b mol/L��10-amol/L��
��4������NaOH��Һ���ȣ��ٽ�ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�����������Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ������������ҺPH��С��
�ʴ�Ϊ����С��
��5����ȫ�к�ʱ����ͼ�����ʵ�����ȣ�a=8�����������Ƶ����ʵ���Ũ��Ϊ0.0001mol/L���к�NaOH��Һ500mL����pH=2��������Һ���=
| 0.0001mol/L��0.5L |
| 0.01mol/L |
�ʴ�Ϊ��5mL��
���������⿼����ˮ��Һ�е����ӻ�����Ӧ�ã���ҺPH����Ӧ�ã��ؼ������ӻ��������¶ȱ仯����Ŀ�ϼ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 �ס�����λͬѧ�����ʵ��ȷ��ij��HA��������ʣ����ڵ���ƽ�⣬�Ҹı�����ƽ�ⷢ���ƶ���ʵ�鷽�����£�
�ס�����λͬѧ�����ʵ��ȷ��ij��HA��������ʣ����ڵ���ƽ�⣬�Ҹı�����ƽ�ⷢ���ƶ���ʵ�鷽�����£�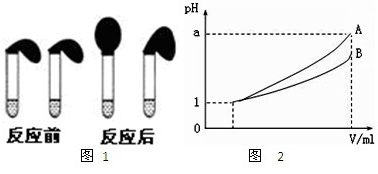

 CH3CH2OH(g)+3H2O(g) ��H��a kJ/mol ��һ��ѹǿ�£����������Ӧ��ʵ���������±���
CH3CH2OH(g)+3H2O(g) ��H��a kJ/mol ��һ��ѹǿ�£����������Ӧ��ʵ���������±���