��Ŀ����
(15��)
����һֱ�������ǹ�ҵ��������Ҫԭ�ϣ��ܳ�һ��ʱ����������Ʒ�����ŷ��������¢�ϡ��ϸ����ͳ��ҹ������Ĺ�ҵ��ѧ�Һ�°���������������ķ����о����ڷ���������ŷ���Ƽ���������Ƽ(�ֳƺ����Ƽ)��������������ҹ������з��ĵ�һ���Ƽ�����Ƽ�ԭ�����������£�
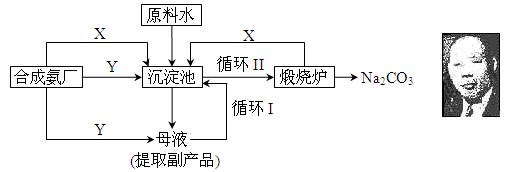
�ź�°�ѡ�������Ϊ�Ƽ�ij�ַ�кα�������(�ٶ���˵��) �� ��
�ƺϳɰ�������Ҫ���Ƽ�ṩ����ԭ�����壬���Ƿֱ��ǣ� �� ��(�ѧʽ)������������ʹ�ù������Ƿ���Ҫ�������ӵ�˳�� (��ǡ���)��ԭ���ǣ� ��
���ڳ������з����ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��ʹԭ��ˮ�����ʵ������ʴ�70%��ߵ�90%���ϣ���Ҫ������� �������������еı�ţ���ѭ������ĸҺ�п�����ȡ�ĸ���Ʒ��Ӧ���� (��һ��)��
����һֱ�������ǹ�ҵ��������Ҫԭ�ϣ��ܳ�һ��ʱ����������Ʒ�����ŷ��������¢�ϡ��ϸ����ͳ��ҹ������Ĺ�ҵ��ѧ�Һ�°���������������ķ����о����ڷ���������ŷ���Ƽ���������Ƽ(�ֳƺ����Ƽ)��������������ҹ������з��ĵ�һ���Ƽ�����Ƽ�ԭ�����������£�

�ź�°�ѡ�������Ϊ�Ƽ�ij�ַ�кα�������(�ٶ���˵��) �� ��
�ƺϳɰ�������Ҫ���Ƽ�ṩ����ԭ�����壬���Ƿֱ��ǣ� �� ��(�ѧʽ)������������ʹ�ù������Ƿ���Ҫ�������ӵ�˳�� (��ǡ���)��ԭ���ǣ� ��
���ڳ������з����ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��ʹԭ��ˮ�����ʵ������ʴ�70%��ߵ�90%���ϣ���Ҫ������� �������������еı�ţ���ѭ������ĸҺ�п�����ȡ�ĸ���Ʒ��Ӧ���� (��һ��)��
��ԭ�Ϸḻ��������� ��1��(���������𰸾���)
��CO2��NH3 ��2��
�� 1��
������ˮ���ܽ�ȴ���ͨ������ͨCO2����̼����臨࣬������̼����������2��
��NaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl 2��
��ѭ��I 2��
������ 2��(���������𰸾���)
��

��ϰ��ϵ�д�
�����Ŀ
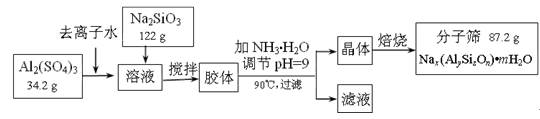
 �ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)�� �� ��
�ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)�� �� �� Ce��
Ce�� Ce��
Ce��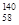 Ce��
Ce��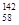 Ce�����ǻ�Ϊͬ��������
Ce�����ǻ�Ϊͬ��������

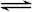 4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���
4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���
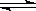 2SO3(g)���о����֣�SO3���������(SO3%)���¶�(T)�ı仯�����ߢ���ʾ�������ж���ȷ����________
2SO3(g)���о����֣�SO3���������(SO3%)���¶�(T)�ı仯�����ߢ���ʾ�������ж���ȷ����________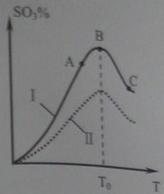
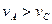
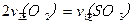

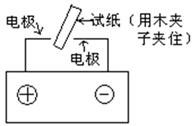
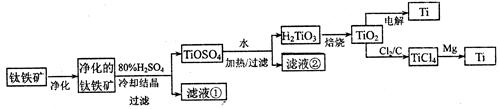
 TICl4��l�� +O2��g��˳�����е�ԭ����:
TICl4��l�� +O2��g��˳�����е�ԭ����: