��Ŀ����
��ǿ���������ڽ�������ʳƷǿ����--�Ҷ��������������Σ��Ҷ��������������λ�ѧʽΪ��
C10Hl2FeN2NaO8��3H2O��ʽ����421��EDTA�����γ�dz����ɫ�ᾧ��ĩ�����ȶ���������ˮ���������Ҵ���l����ˮ��ҺpHԼΪ3.5�������Ҷ���������һ���Σ�NaH3EDTA�����Ȼ�����ȡ����ȡԭ������
C10Hl2FeN2NaO8��3H2O��ʽ����421��EDTA�����γ�dz����ɫ�ᾧ��ĩ�����ȶ���������ˮ���������Ҵ���l����ˮ��ҺpHԼΪ3.5�������Ҷ���������һ���Σ�NaH3EDTA�����Ȼ�����ȡ����ȡԭ������

ʵ�����Ʊ�NaFeEDTA��3H2O�������£�
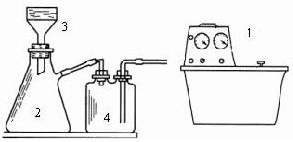
�ٰ�0��4g��0��0lmol��NaOH����l0mL����ˮ��Ȼ���ټ���3��8g��0��01mo1��Na2H2EDTA��2H2O������Һ�ȣ�ֱ��������ȫ�ܽ⡣
���ú����Ȼ�ͭ���ʵ��Ȼ����ᴿ���Ƶ�FeCl3��6H2O����ȡ2��5g��0��009mol��FeCl3��6H2O����5mL������ˮ�У�����l�����ᣩ��
�۽��١����Ƶõ�����Һ���
�ܼ�����Һ�����ڣ�����Ũ��ֱ���ַ�ĩ״�Ĺ���������
����ȴ����ѹ���ˣ����ñ�ˮϴ�������Ҵ�ϴ�ӡ�
���������ɣ��Ƶ��Ƶõĵ���ɫ��ĩ2��8g
�Իش���������
��1���ڲ�����У����������ļ����Ϊ_________________��
��2���������Ȼ�ͭ���ʵ��Ȼ�����Һ������õļ�����___________________________������ɫ����ֱ�����Ӧ�����ӷ���ʽΪ_______________________��_________________________��
��3����������ñ�ˮ���Ҵ�ϴ�ӵ�Ŀ���ǣ�_________________��_________________��
��4����ʵ��õ����Ƿ�ĩ״����ɫ�ķ�ĩ��û�еõ���״�ľ��壬���ܵ�ԭ���ǣ�__________________________________��
��5������ijƷ�ƽ������Ƿ����NaFeEDTA��3H2O����ȡ20mL���ͼ���10rnL75���Ҵ��������ó���������ˮ������ij�������pH<0��5���ƻ�����Ȼ�����ij����μ��飬�������_________________��д��ѧʽ����
��6������ʵ���NaFeEDTA��3H2O��ʵ�ʲ���Ϊ��_________________��
�ٰ�0��4g��0��0lmol��NaOH����l0mL����ˮ��Ȼ���ټ���3��8g��0��01mo1��Na2H2EDTA��2H2O������Һ�ȣ�ֱ��������ȫ�ܽ⡣
���ú����Ȼ�ͭ���ʵ��Ȼ����ᴿ���Ƶ�FeCl3��6H2O����ȡ2��5g��0��009mol��FeCl3��6H2O����5mL������ˮ�У�����l�����ᣩ��
�۽��١����Ƶõ�����Һ���
�ܼ�����Һ�����ڣ�����Ũ��ֱ���ַ�ĩ״�Ĺ���������
����ȴ����ѹ���ˣ����ñ�ˮϴ�������Ҵ�ϴ�ӡ�
���������ɣ��Ƶ��Ƶõĵ���ɫ��ĩ2��8g
�Իش���������
��1���ڲ�����У����������ļ����Ϊ_________________��
��2���������Ȼ�ͭ���ʵ��Ȼ�����Һ������õļ�����___________________________������ɫ����ֱ�����Ӧ�����ӷ���ʽΪ_______________________��_________________________��
��3����������ñ�ˮ���Ҵ�ϴ�ӵ�Ŀ���ǣ�_________________��_________________��
��4����ʵ��õ����Ƿ�ĩ״����ɫ�ķ�ĩ��û�еõ���״�ľ��壬���ܵ�ԭ���ǣ�__________________________________��
��5������ijƷ�ƽ������Ƿ����NaFeEDTA��3H2O����ȡ20mL���ͼ���10rnL75���Ҵ��������ó���������ˮ������ij�������pH<0��5���ƻ�����Ȼ�����ij����μ��飬�������_________________��д��ѧʽ����
��6������ʵ���NaFeEDTA��3H2O��ʵ�ʲ���Ϊ��_________________��
��1���������ʹ�������������������ɣ�Ӱ�촿��
��2��ֽ�ϲ�����Fe3++3NH3��H2O=Fe(OH)3��+3NH4+��Cu2++4NH3��H2O=Cu(NH3)42++4H2O
��3��ϴ�ӳ�ȥ������渽�ŵ�Na2H2EDTA���Ȼ�������ʣ��ñ�ˮϴ�ӿɽ���ϴ�ӹ�����
NaFeEDTA��3H2O����ģ��þƾ�ϴ�ӿ�ʹ����ٸ���
��4����������ܼ������ٶȿ죬�������ȴ���ʿ�
��5��NH4SCN
��6��78.9��
��2��ֽ�ϲ�����Fe3++3NH3��H2O=Fe(OH)3��+3NH4+��Cu2++4NH3��H2O=Cu(NH3)42++4H2O
��3��ϴ�ӳ�ȥ������渽�ŵ�Na2H2EDTA���Ȼ�������ʣ��ñ�ˮϴ�ӿɽ���ϴ�ӹ�����
NaFeEDTA��3H2O����ģ��þƾ�ϴ�ӿ�ʹ����ٸ���
��4����������ܼ������ٶȿ죬�������ȴ���ʿ�
��5��NH4SCN
��6��78.9��

��ϰ��ϵ�д�
 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д�
�����Ŀ
�����и����ʵ��������ж���ʵ�ʳɷֵ�˵������ȷ���ǣ�������
| A����������һ�����͵ĺϽ���� | B���־�������һ�����ʵ����Ͻ�����ģ�����ѹ���ܷdz��ã������Ƴɸ�ѹ�� | C��ů���ܵ��Լ�ů��Ƭ�ȱ���ˢ��һ�㡰���ۡ�����ʵ�������� | D���������;����ڽ����м���������������� |
���ѧ���Ļ�ѧ֪ʶ���ж�����������ȷ���� �� ��
| A��������Ư�۳���������ˮ�ľ�����ɱ�����������ߵ�����ԭ����ͬ |
| B���ճ���������ˮ�Ҵ�������ɱ������ |
| C�������ЧӦ�����ֽ������Һ��һ�ֳ����������� |
| D���������;����ڽ����м���������������ۣ����ҹ��IJ������� |
 �й�����Ԥ���������ĵĵ�����ʾ���й���ͯƶѪ����25%���ң���ŮƶѪ����20%���ң���������ƶѪ������10%���ң��ƹ���ǿ�������ܹ�������ȱ����ȱ����ƶѪ���ı�Ŀǰ�й���Ⱥ��ȱ����״����ǿ���������ڽ����м���һ�������Ҷ������������ƣ�NaFeEDTA���Ƴɵ�Ӫ��ǿ����ζƷ���Ҷ��������ᣨEDTA���Ľṹ��ʽ��ͼ��ʾ�������й�˵������ȷ���ǣ�������
�й�����Ԥ���������ĵĵ�����ʾ���й���ͯƶѪ����25%���ң���ŮƶѪ����20%���ң���������ƶѪ������10%���ң��ƹ���ǿ�������ܹ�������ȱ����ȱ����ƶѪ���ı�Ŀǰ�й���Ⱥ��ȱ����״����ǿ���������ڽ����м���һ�������Ҷ������������ƣ�NaFeEDTA���Ƴɵ�Ӫ��ǿ����ζƷ���Ҷ��������ᣨEDTA���Ľṹ��ʽ��ͼ��ʾ�������й�˵������ȷ���ǣ�������