题目内容
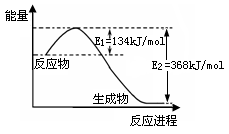
已知: 3/2CO2(g)+2Fe(s) = Fe2O3(s)+3/2C(s) △H=-234.1kJ·mol-1
CO2(g) = C(s)+O2(g) △H=+393.4kJ·mol-1。
则2Fe(s)+3/2O2(g)=Fe2O3(s)的△H是
CO2(g) = C(s)+O2(g) △H=+393.4kJ·mol-1。
则2Fe(s)+3/2O2(g)=Fe2O3(s)的△H是
| A.-824.2kJ·mol-1 | B.-627.5kJ·mol-1 |
| C.+861.6kJ·mol-1 | D.+159.3kJ·mol-1 |
A
CO2(g) = C(s)+O2(g) △H=+393.4kJ·mol-1知C(s)+O2(g)= CO2(g) △H=-393.4kJ·mol-1则3/2C(s)+3/2O2(g)="3/2" CO2(g) △H=-393.4kJ·mol-1*3/2=-590.1kJ·mol-1该式与所给一式相加得2Fe(s)+3/2O2(g)=Fe2O3(s) △H=-824.2kJ·mol-1故选A。

练习册系列答案
 阶梯计算系列答案
阶梯计算系列答案
相关题目
 2NH3(g)+CO2(g) 在不同温度下,该反应平衡状态部分数据见右表。下列说法正确的是
2NH3(g)+CO2(g) 在不同温度下,该反应平衡状态部分数据见右表。下列说法正确的是 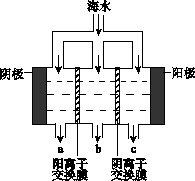 电渗析法淡化海水示意图如下图所示,其中阴(阳)离子交换膜仅允许阴(阳)离子通过。
电渗析法淡化海水示意图如下图所示,其中阴(阳)离子交换膜仅允许阴(阳)离子通过。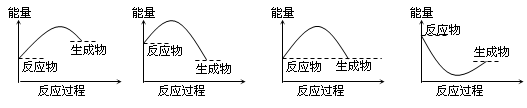
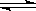 SO3(g) △H = ―98.32kJ/mol,在容器中充入2molSO2 和1molO2充分反应,最终放出的热量为
SO3(g) △H = ―98.32kJ/mol,在容器中充入2molSO2 和1molO2充分反应,最终放出的热量为