��Ŀ����
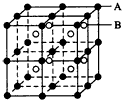
(1)��A��B��Ԫ����ɵ����Ӿ���ṹ���ף���ͼ��ʾ����þ���Ļ�ѧʽ�ǣ�___________��
(2)Cԭ�ӵ����������Ų�Ϊnsnnpm����ijһ����̬��ʹ�������Dz��ÿһ�����������ȫ��״̬��1��Cԭ����2��Dԭ�ӽ�ϣ�ʹ˫�����ﵽ8�����ȶ��ṹ������C��D�γɵķ����У�Cԭ�ӷ���������ӻ���ʽ��________�����ӹ�����________���ӳɼ���ʽ�����÷����к��еĹ��ۼ������ͺ���Ŀ�ֱ���________________
(3)C��D�γɵij������壨�ң����������۵���ȣ���____�ף���ԭ����___________________��
(3)C��D�γɵij������壨�ң����������۵���ȣ���____�ף���ԭ����___________________��
(1)AB��BA
(2)sp��ֱ���Σ�2���Ҽ���2���м�
(3)<��������̼�������̼������Ϊ���Ӿ��壬�������������ķ��Ӽ�����������AB����Ϊ���Ӿ��壬��������Ϊ��ǿ�����Ӽ�
(2)sp��ֱ���Σ�2���Ҽ���2���м�
(3)<��������̼�������̼������Ϊ���Ӿ��壬�������������ķ��Ӽ�����������AB����Ϊ���Ӿ��壬��������Ϊ��ǿ�����Ӽ�

��ϰ��ϵ�д�
�����Ŀ
 A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������A��C��B��D�ֱ���ͬ����Ԫ�أ���֪B��D��Ԫ��ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵�2��������Ԫ���γɵĵ����������������壬�����ǹ��壮��ش��������⣺
A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������A��C��B��D�ֱ���ͬ����Ԫ�أ���֪B��D��Ԫ��ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵�2��������Ԫ���γɵĵ����������������壬�����ǹ��壮��ش��������⣺
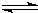 NH4++OH-
NH4++OH-