��Ŀ����
ͭ���仯�����ڹ�ҵ�������ճ����������Ź㷺��Ӧ�á�
(1) Li--CuO����ܷ�ӦΪ��2Li + CuO = Li2O + Cu���������ҺΪ��������л���Һ����ص�������ӦʽΪ _____________��
(2) Cu2O���ְ뵼����ϣ����ڼ�����Һ���õ�ⷨ��ȡ������ܷ�ӦʽΪ��2Cu + H2O Cu2O + H2����������ӦʽΪ_________��Cu2OͶ��ϡ�����У��õ���ɫ��Һ�ͺ�ɫ������д���÷�Ӧ�����ӷ���ʽ___________________��
Cu2O + H2����������ӦʽΪ_________��Cu2OͶ��ϡ�����У��õ���ɫ��Һ�ͺ�ɫ������д���÷�Ӧ�����ӷ���ʽ___________________��
(3)���Ȼ�����Һ��ʴӡˢ��·�ְ��ķ�Һ�У��������������ʵ���Ũ�ȵĶ���ֵ����ҺpH�ı仯����ͼ��ʾ(����Һ�н�������Ũ�ȡ�10�C5 mol��L �C1ʱ������Ϊ������ȫ)��
(1) Li--CuO����ܷ�ӦΪ��2Li + CuO = Li2O + Cu���������ҺΪ��������л���Һ����ص�������ӦʽΪ _____________��
(2) Cu2O���ְ뵼����ϣ����ڼ�����Һ���õ�ⷨ��ȡ������ܷ�ӦʽΪ��2Cu + H2O
 Cu2O + H2����������ӦʽΪ_________��Cu2OͶ��ϡ�����У��õ���ɫ��Һ�ͺ�ɫ������д���÷�Ӧ�����ӷ���ʽ___________________��
Cu2O + H2����������ӦʽΪ_________��Cu2OͶ��ϡ�����У��õ���ɫ��Һ�ͺ�ɫ������д���÷�Ӧ�����ӷ���ʽ___________________�� (3)���Ȼ�����Һ��ʴӡˢ��·�ְ��ķ�Һ�У��������������ʵ���Ũ�ȵĶ���ֵ����ҺpH�ı仯����ͼ��ʾ(����Һ�н�������Ũ�ȡ�10�C5 mol��L �C1ʱ������Ϊ������ȫ)��
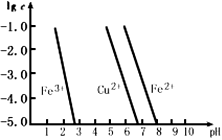
����ͼ��֪Fe(OH)2��Cu(OH)2���ܶȻ�Ksp[Fe(OH)2]________Ksp[Cu(OH)2](�>����<��)������Һ��Cu2+��Fe3+��Fe2+Ũ�Ⱦ�Ϊ0.1 mol/L��������ͨ�백��������Һ��pH = 5.6ʱ����Һ�д��ڵĽ���������Ϊ________________��
(4)����ͭ�۶Ը�����淋��ȷֽ��д����ã�1 mol������立ֽ�ʱ������2 molˮ������1 mol�����⣬�������Ԫ�ؾ��γ���̬���ʡ��÷�Ӧ�Ļ�ѧ����ʽΪ_________________��1 mol������立ֽ���������������________mol��
(4)����ͭ�۶Ը�����淋��ȷֽ��д����ã�1 mol������立ֽ�ʱ������2 molˮ������1 mol�����⣬�������Ԫ�ؾ��γ���̬���ʡ��÷�Ӧ�Ļ�ѧ����ʽΪ_________________��1 mol������立ֽ���������������________mol��
(1)CuO �C 2e�C + 2Li+ = LiO + Cu
(2)2Cu �C 2e�C + 2OH�C = Cu2O + H2O��Cu2O + 2H+ = Cu2+ + H2O
(3) >��Cu2+��Fe2+
(4)2NH4ClO4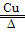 4H2O + 2O2�� + N2�� + Cl2����4
4H2O + 2O2�� + N2�� + Cl2����4
(2)2Cu �C 2e�C + 2OH�C = Cu2O + H2O��Cu2O + 2H+ = Cu2+ + H2O
(3) >��Cu2+��Fe2+
(4)2NH4ClO4
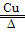 4H2O + 2O2�� + N2�� + Cl2����4
4H2O + 2O2�� + N2�� + Cl2����4 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
