��Ŀ����
19�������ϱ������Ҷ��ᣨHOOC-COOH�����ɼ�дΪH2C2O4���׳Ʋ��ᣬ��һ�ְ�ɫ���壬������ˮ�����۵�Ϊ101.5�棬��157�����������ڶ�Ԫ���ᣮ����β�����ˮ��ijУ��ѧ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ���������ͼ1ʵ�飺
��1��Ϊ�Ƚ���ͬŨ�ȵIJ��������ĵ����ԣ�ʵ����������100mL 0.1mol•L-1�IJ�����Һ������ �������õ��IJ�����������Ͳ���ձ�����������100mL����ƿ����ͷ�ιܣ�
��2����֪�������ƣ�NaHC2O4����Һ�����ԣ�
�������ӷ���ʽ����NaHC2O4��Һ�����Ե�ԭ��HC2O4-?H++C2O42-
�ڳ�����0.1mol•L-1 Na2C2O4��Һ�и�������Ũ�ȵĴ�С��ϵc��Na+����c��C2O42-����c��OH-����c��HC2O4-����c��H+��
��3����Ϊ̽���������ȷֽ�IJ����ͬѧ������������ƣ�
ʵ���У��۲쵽��ˮ����ͭ����������ʯ��ˮ����ǣ��ڸ���ܼ��촦��ȼ�ݳ������壬�ձ� �ڱڸ��еij���ʯ��ˮ����ǣ�֤����������H2O��CO2��CO
����ͬѧ��Ϊ����ͼ2��ʾ����������ɵ�װ���ܸ��õ���֤�������
��װ�õ�����˳��ΪACDBE������ţ�Cװ�õ�������ʹ����������������ֹ���Ŷ�CO2�ļ��飮
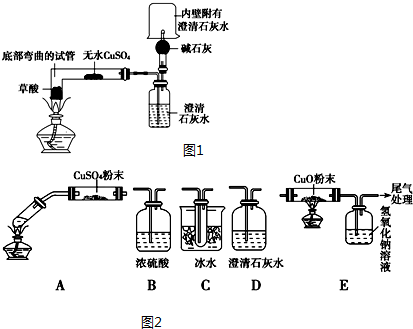
���� ��1��������Һ���Ʋ�����̷�����Ҫ�IJ���������
��2���ٲ������ƣ�NaHC2O4����Һ�������Dz���������ӵ������ˮ�⣻
�ڸ�����Һ��������ӵ�ˮ��ȷ����Һ�и������ӵ�Ũ�ȹ�ϵ��
��3�������ݷ�Ӧ��������жϲ���ֽ����ɵIJ����ˮ����ͭ����˵������ˮ������ʯ��ˮ�����ʲô���ɶ�����̼���ڸ���ܼ��촦��ȼ�ݳ������壬�ձ��ڱڸ��еij���ʯ��ˮ�����˵������һ����̼��
�ڷ���װ��ͼ���Լ���֪װ��A�Ƿֽ���ᣬͨ����ˮ����ͭ�������������к���ˮ������ͨ��װ��C����������������ֹ���Ŷ�����̼����ļ��飬ͨ��װ��D���������̼�Ĵ��ڣ�װ��B�dz�ȥˮ������ͨ��������ͭ�����Ƿ�����һ����̼����Ӧ������ͨ��װ��E�еij���ʯ��ˮ�����˵������һ����̼������ȼʣ�����壮
��� �⣺��1��Ϊ�Ƚ���ͬŨ�ȵIJ��������ĵ����ԣ�ʵ����������100L 0.1mol•L-1�IJ�����Һ�����ƹ������õ��IJ�����������Ͳ���ձ�����������100m L����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��2���ٲ������ƣ�NaHC2O4����Һ�������Dz���������ӵ������ˮ�⣬���뷽��ʽΪ��HC2O4-?H++C2O42-���ʴ�Ϊ��HC2O4-?H++C2O42-��
��Na2C2O4��Һ�������Ӳ�ˮ�⣬C2O42-ˮ������HC2O4-��OH-��HC2O4-ˮ������H2C2O4��OH-������Na2C2O4��Һ�ʼ��ԣ���Һ�и�������Ũ�ȵĴ�С��ϵΪ��c��Na+����c��C2O42-����c��OH-����c��HC2O4-����c��H+�����ʴ�Ϊ��c��Na+����c��C2O42-����c��OH-����c��HC2O4-����c��H+����
��3����ʵ���У��۲쵽��ˮ����ͭ����˵���ֽ������к���ˮ����������ʯ��ˮ�����˵���ֽ����ɵ������к��ж�����̼���ڸ���ܼ��촦��ȼ�ݳ������壬�ձ��ڱڸ��еij���ʯ��ˮ����ǣ�˵���ֽ������к���һ����̼��֤����������H2O��CO2��CO���ʴ�Ϊ��H2O��CO2��CO��
�ڷ���װ��ͼ���Լ���֪װ��A�Ƿֽ���ᣬͨ����ˮ����ͭ�������������к���ˮ������ͨ��װ��C����������������ֹ���Ŷ�����̼����ļ��飬ͨ��װ��D���������̼�Ĵ��ڣ�װ��B�dz�ȥˮ������ͨ��������ͭ�����Ƿ�����һ����̼����Ӧ������ͨ��װ��E�еij���ʯ��ˮ�����˵������һ����̼������ȼʣ�����壬װ�õ�����˳��ΪACDBE��
�ʴ�Ϊ��ACDBE��ʹ����������������ֹ���Ŷ�CO2�ļ��飮
���� ���⿼����������ɵ�ʵ��̽�������Ͳ����ʵ�鹤�������жϣ������������ʺ�װ������������ʵ��������������ʵ������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�| A�� | Σ�ջ�ѧƷ���Լ�ƿ��ǩ�����װ��һ�㶼�������ʶ | |
| B�� | �����κ��Լ����ɼ���AlCl3��Һ��NaOH��Һ | |
| C�� | ��ʹ����CuO��������һ����H2 | |
| D�� | �������ױ�����ˮ�� |
| A�� | ��� | B�� | ���� | ||
| C�� | ���ʵ���Ũ�� | D�� | H+��OH-�����ʵ��� |
| A�� | ��״���£�2.24 L CCl4�к��еķ���������0.1NA | |
| B�� | �����£�1 mol/L Na2CO3��Һ����������������0.1NA | |
| C�� | ������ΪNA��C2H4�������ԼΪ22.4 L��������һ���DZ�״�� | |
| D�� | ��״���£�4.2 g C3H6�к��е�̼̼˫����һ��Ϊ0.1 NA |
| A�� | 2 | B�� | 4 | C�� | 8 | D�� | 9 |
�پ���ϩ����ϩ�� �ڼ�ȩ�������ǡ� �۵��ۺ���ά�آ����Ǻ���ѿ�ǡ��ݾ���ϩ�;�����ϩ��
| A�� | �٢ڢܢ� | B�� | �٢ڢ� | C�� | �٢ۢܢ� | D�� | �٢ڢۢ� |
| A�� | һ���㣺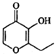 | B�� | �ұصã�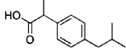 | ||
| C�� | ά����B5��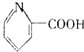 | D�� | ����Ϣʹ��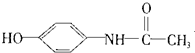 |
| A�� | FeCl3 | B�� | FeCl2 | C�� | Fe��OH��3 | D�� | Fe3O4 |