��Ŀ����
14��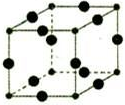 Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����ã�
Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����ã���1����N3-������ͬ����������ԭ�ӷ��ӵĿռ乹����V�Σ�
��2��Cu�������õĵ��硢���Ⱥ���չ�ԣ������Cu���е����Ե�ԭ��CuΪ�������壬�����д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
��3����Cu�Ĵ������£��Ҵ��ɱ���������Ϊ��ȩ����ȩ������̼ԭ�ӵ��ӻ���ʽ��sp3��sp2����ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ��ǣ�����ڡ��������ڡ���С�ڡ�����
��4��Cu+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d10������������Һ�в��ȶ����ɷ����绯��Ӧ����Cu2+��Cu����CuO�ڸ����»�ֽ��Cu2O���Դӽṹ�ǶȽ�������CuOΪ�λ�����Cu2OCu+��3d����ϵ���ȫ����ṹ�ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ���Ի���[Cu��H2O��2��Cl��2]���м��Եķ��ӵĽṹʽ
 ��
����6��Cu3N�ľ����ṹ��ͼ��N3-����λ��Ϊ6��Cu+�뾶Ϊapm��N3-�뾶Ϊb pm��Cu3N���ܶ�$\frac{103��10{\;}^{30}}{4��a+b��{\;}^{3}N{\;}_{A}}$g/cm3���������ӵ���Ϊ������NA��ʾ��
���� ��1����N3-������ͬ����������Ϊ�ȵ����壬��NO2-���ȵ�����ṹ���ƣ����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
��2���������ɵ��ӵĽ��������ܵ��磻
��3����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ����ݴ��ж�̼ԭ�ӵ��ӻ���ʽ��̼ԭ���ӻ���ʽ��ͬ��������Dz�ͬ��
��4��Cu+�ĺ�����28�����ӣ����ݹ���ԭ����д���̬���Ӻ�������Ų�ʽ��ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu��H2O��2��Cl��2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�
��6��Cu3N�ľ����ṹ��ͼ���������=12��$\frac{1}{4}$=3��С�����=$\frac{1}{8}��8$=1�����Դ����ʾCuԭ�ӡ�С���ʾNԭ�ӣ�N3-����λ��=3��2=6��Cu3N���ܶ�=$\frac{m}{V}$��
��� �⣺��1����N3-������ͬ����������Ϊ�ȵ����壬��NO2-���õ�����ṹ���ƣ��������������Nԭ�Ӽ۲���ӶԸ���=2+$\frac{1}{2}$����5+1-2��2��=3�Һ���һ���µ��Ӷԣ�����ΪV�νṹ��
�ʴ�Ϊ��V�Σ�
��2��ͭ���ڽ������壬�����к��п��������ƶ��ĵ��ӣ�ͨ������ƶ��������ܵ��磬
�ʴ�Ϊ��CuΪ�������壬�����д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
��3����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ������Լ��е�̼ԭ�Ӳ���sp3�ӻ���ȩ���е�̼ԭ�Ӳ���sp2�ӻ���ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ���������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ��ǣ�
�ʴ�Ϊ��sp3��sp2�����ڣ�
��4��Cu+�ĺ�����28�����ӣ����ݹ���ԭ��֪���̬���Ӻ�������Ų�ʽ1s22s22p63s23p63d10��ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ���Cu+��3d�����ȫ�����ȶ���
�ʴ�Ϊ��1s22s22p63s23p63d10��Cu+��3d����ϵ���ȫ����ṹ�ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu��H2O��2��Cl��2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�����ṹʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��Cu3N�ľ����ṹ��ͼ���������=12��$\frac{1}{4}$=3��С�����=$\frac{1}{8}��8$=1�����Դ����ʾCuԭ�ӡ�С���ʾNԭ�ӣ�N3-����λ��=3��2=6�����������=[��2a+2b����10-10cm]3��Cu3N���ܶ�=$\frac{m}{V}$=$\frac{\frac{64��3+14}{N{\;}_{A}}}{[��2a+2b����10{\;}^{-10}]{\;}^{3}}$g/cm3=$\frac{103��10{\;}^{30}}{4��a+b��{\;}^{3}N{\;}_{A}}$g/cm3��
�ʴ�Ϊ��6��$\frac{103��10{\;}^{30}}{4��a+b��{\;}^{3}N{\;}_{A}}$��
���� ���⿼�������ʽṹ�����ʣ��漰�����ļ��㡢ԭ���ӻ�����������Ų���֪ʶ�㣬�����ܶȹ�ʽ���۲���ӶԻ������ۡ�����ԭ����֪ʶ�������������Щ֪ʶ�㶼�ǿ����ȵ㣬�ѵ��Ǿ����ļ��㣬��ȷ������ĸ�ĺ��壬ע�⣨1���в���֪ʶǨ�Ƶķ������н����Ŀ�Ѷ��еȣ�

| A�� | �����ձ��ײ��Ĵ��ζ� | |
| B�� | ��ͣ���ڱ�����������ų� | |
| C�� | ���ڱ���Һ���Ϸ�Ӧ���Ĵ��ζ� | |
| D�� | ���ڱ�����ˮ��Ľ��紦���������������ݲ��� |
| A�� | 3��2 | B�� | 2��3 | C�� | 1��1 | D�� | 5��4 |
K Na KCl NaCl?
�۵㣨�棩 63.6 97.8 770 801?
�е㣨�棩 774 882.9 1500 1413?
����ƽ���ƶ�ԭ��������֪��Na��KCl��Ӧ��ȡ�����ص������¶���?��������
| A�� | ����770�� | B�� | 850�� | C�� | ����882.9�� | D�� | 1413��1500�� |
��CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ•mol-1
��CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1 160kJ•mol-1
����˵������ȷ���ǣ�������
| A�� | ���ñ�״����4.48L CH4��ԭNO2����N2��ˮ�������ų�������Ϊ173.4kJ | |
| B�� | �ɷ�Ӧ�ٿ���֪��CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��l����H��-574kJ•mol-1 | |
| C�� | ��Ӧ�٢�ת�Ƶĵ�������ͬ | |
| D�� | ��Ӧ���е�4.48L����״����CH4��Ӧ��ȫʱת�Ƶĵ��ӵ����ʵ���Ϊ1.6mol |
| A�� |  | B�� |  | C�� |  | D�� | 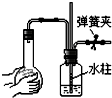 |
| A�� | ��ͼ���һ��ʱ�����Һ��ɫ��dz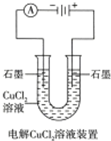 | B�� | ��ͼ��������Ʒ���Դ��������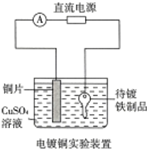 | ||
| C�� | ��ͼ������b������a��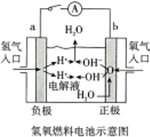 | D�� | ��ͼ������Ӧʽ��Ix-+��x-1��e-=xI- |
