��Ŀ����
(1)��֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1 mol/L������5����Һ��pH: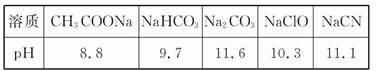
����������Ϣ�жϣ�Ũ�Ⱦ�Ϊ0.05 mol/L������5�����ʵ���Һ�У�pH��С���� (����);��pHΪ (����ֵ)��pH������ (����)��
��CH3COOH ��HCN ��HClO ��H2SO4 ��HClO4
(2)����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ��
���ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ��塣
������KCl��NaNO3�����Һ����������NaCl���塣
����������Ӧ���ܽ�����ֽⷴӦ������һ�����ɣ� ��
��KI��Һ��AgCl�����Ͻ��裬��۲쵽�������� ,
��д����Ӧ�����ӷ���ʽ�� ��
(1)�� 1 ��
����

�±��Dz�ͬ�¶���ˮ�����ӻ������ݣ�
| �¶�/�� | 25 | t1 | t2 |
| ˮ�����ӻ� | 1��10��14 | a | 1��10��12 |
�Իش��������⣺
(1)��25��t1��t2����a________1��10��14(�����������������)�������жϵ�������_______________________________________________________��
(2)25 ��ʱ��ijNa2SO4��Һ��c(SO42��)��5��10��4 mol��L��1��ȡ����Һ1 mL��ˮϡ����10 mL����ϡ�ͺ���Һ��c(Na��)��c(OH��)��________��
(3)��t2�¶��²��ij��ҺpH��7������Һ��________(��ᡱ��������С�)�ԣ������¶���pH��11��NaOH��Һa L��pH��1��H2SO4��Һb L��ϣ������û��ҺpH��2����a��b��________��
�Դ�����п�ۣ���FeO��Fe2O3��ZnS�ȣ���ȡ����ZnO�Ĺ������£�
����1����H2SO4����������п��ͬʱ����H2O2��
����2�����ˣ�������Һ��pH��
����3�����ˣ�����Һ�м�NH4HCO3���ü�ʽ̼��п������
����4�����ˡ�ϴ�ӡ����գ��ò�Ʒ��
��֪�����ӳ�����pH���±���
| ���� | ��ʼ����pH | ������ȫpH |
| Fe2+ | 7.6 | 9.6 |
| Fe3+ | 2.7 | 3.7 |
| Zn2+ | 5.4 | 8.0 |
��1������H2O2ʱ�ܽ�����п��ͬʱ���ɵ���ɫ���壬д���仯ѧ����ʽ ��
��2������2�е�����ҺpH�ķ�Χ�� ��
��3��ȡϴ�ӡ������ļ�ʽ̼��п68.2 g��������պ��ò������ʵ�����Ϊ48.6 g������������ͨ����������ʯ��ˮ�У��ó���20 g�������ʽ̼��п����ɣ��û�ѧʽ��ʾ��д��������̣���
һ�ֺ�����ﮡ��ܵ����͵��Ӳ��ϣ������в����ķ��������ɹۣ������е����Խ�����������ʽ���ڣ�����Co2O3��CoO����ʽ���ڣ������������ĵ����˫�棻﮻��������С�
�ӷ����л��������ܣ�CoO���Ĺ����������£�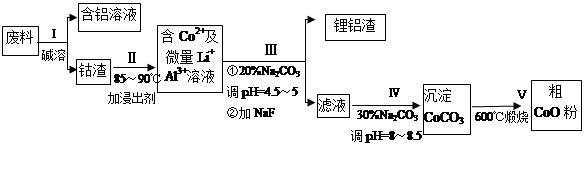
��1������I�в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ ��
��2�����̢�õ����������Ҫ�ɷ���LiF��Al(OH)3��̼������Һ�ڲ���Al(OH)3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ________ ____________��
��3��̼������Һ�ڹ���III��IV����������������ͬ����д���ڹ���IV����������� ��
��4����Na2CO3��Һ�д��ڶ������ӣ����и�����Ũ�ȹ�ϵ��ȷ����___ ___������ţ���
| A��c(Na+) = 2c(CO32-) |
| B��c(Na+) > c(HCO3-) > c(CO32-) |
| C��c(OH-) > c(HCO3-) > c(H+) |
| D��c(OH-) - c(H+) = c(HCO3-) + 2c(H2CO3) |
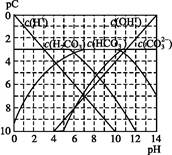
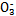 ����ʹ����ѪҺpH������7.35~7.45�����á������õ������Һ�е�ƽ�����:������������������������������(�����ӷ���ʽ��ʾ)��
����ʹ����ѪҺpH������7.35~7.45�����á������õ������Һ�е�ƽ�����:������������������������������(�����ӷ���ʽ��ʾ)�� 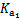 ������������
������������ 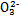 ��������������10%��������Ƽ�ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ
��������������10%��������Ƽ�ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ  ��S
��S
 H++OH-��KW=10-14
H++OH-��KW=10-14 b(����ڡ���С�ڡ����ڡ�)����a��b��ʾNH3��H2O�ĵ���ƽ�ⳣ��Ϊ����������
b(����ڡ���С�ڡ����ڡ�)����a��b��ʾNH3��H2O�ĵ���ƽ�ⳣ��Ϊ����������  H����A2����
H����A2����