��Ŀ����
һˮ�����İ���ͭ(��)([Cu(NH3)4]SO4��H2O)��һ����Ҫ��Ⱦ�ϼ�ũҩ�м��塣��ش������������:
(1)Cu�ĺ�������Ų�ʽΪ ��
(2)N��L������ �ԳɶԵ���;N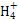 ���幹���� ,������ԭ�ӵ��ӻ���ʽΪ ��
���幹���� ,������ԭ�ӵ��ӻ���ʽΪ ��
(3)��ˮ��Һ�д��ڶ������,�α�ʾ���������� ;
(4)[Cu(NH3)4]SO4��H2O�г�����ɫ�������� ,�����еġ����ӶԸ���һ���ܼ������� ��;
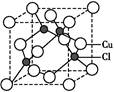
(5)Cu��һ���Ȼ��ᄃ���ṹ��ͼ��ʾ,���Ȼ���Ļ�ѧʽ ��
(1)Cu�ĺ�������Ų�ʽΪ ��
(2)N��L������ �ԳɶԵ���;N
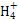 ���幹���� ,������ԭ�ӵ��ӻ���ʽΪ ��
���幹���� ,������ԭ�ӵ��ӻ���ʽΪ ��(3)��ˮ��Һ�д��ڶ������,�α�ʾ���������� ;
(4)[Cu(NH3)4]SO4��H2O�г�����ɫ�������� ,�����еġ����ӶԸ���һ���ܼ������� ��;
(5)Cu��һ���Ȼ��ᄃ���ṹ��ͼ��ʾ,���Ȼ���Ļ�ѧʽ ��

(1)1s22s22p63s23p63d104s1
(2)1 �������� sp3
(3)O��H��N N��H��O(��O��H��O)
(4)[Cu(NH3)4]2+ ��λ(��)
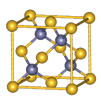
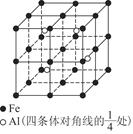
(5)CuCl
(2)1 �������� sp3
(3)O��H��N N��H��O(��O��H��O)
(4)[Cu(NH3)4]2+ ��λ(��)
(5)CuCl
(1)Cu�ڵ�4���ڢ�B��,�����Ų�Ϊ1s22s22p63s23p63d104s1;(2)N�ĵ����Ų�ʽΪ1s22s22p3,L���Ӳ㼴�ڶ��ܲ�,���ֻ��1�ԳɶԵ���,N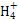 ����ԭ�ӵ�ԭ�Ӳ���sp3�ӻ�,�ռ乹��Ϊ����������;(3)��ˮ��Һ�а������ӺͰ�������֮������γ����,ˮ���Ӻ�ˮ����֮������γ����,ˮ���ӺͰ�������֮��Ҳ�����γ����(������ʽ),���ͨ���á�������ʾ;(4)������[Cu(NH3)4]2+ʹ��Һ������ɫ,�������к�����λ��;(5)��̯��,�þ����к���ͭԭ��8��
����ԭ�ӵ�ԭ�Ӳ���sp3�ӻ�,�ռ乹��Ϊ����������;(3)��ˮ��Һ�а������ӺͰ�������֮������γ����,ˮ���Ӻ�ˮ����֮������γ����,ˮ���ӺͰ�������֮��Ҳ�����γ����(������ʽ),���ͨ���á�������ʾ;(4)������[Cu(NH3)4]2+ʹ��Һ������ɫ,�������к�����λ��;(5)��̯��,�þ����к���ͭԭ��8�� +6��
+6�� =4��,��ԭ��4��,������1��1�Ĺ�ϵ,��˸��Ȼ���Ļ�ѧʽΪCuCl��
=4��,��ԭ��4��,������1��1�Ĺ�ϵ,��˸��Ȼ���Ļ�ѧʽΪCuCl��
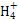 ����ԭ�ӵ�ԭ�Ӳ���sp3�ӻ�,�ռ乹��Ϊ����������;(3)��ˮ��Һ�а������ӺͰ�������֮������γ����,ˮ���Ӻ�ˮ����֮������γ����,ˮ���ӺͰ�������֮��Ҳ�����γ����(������ʽ),���ͨ���á�������ʾ;(4)������[Cu(NH3)4]2+ʹ��Һ������ɫ,�������к�����λ��;(5)��̯��,�þ����к���ͭԭ��8��
����ԭ�ӵ�ԭ�Ӳ���sp3�ӻ�,�ռ乹��Ϊ����������;(3)��ˮ��Һ�а������ӺͰ�������֮������γ����,ˮ���Ӻ�ˮ����֮������γ����,ˮ���ӺͰ�������֮��Ҳ�����γ����(������ʽ),���ͨ���á�������ʾ;(4)������[Cu(NH3)4]2+ʹ��Һ������ɫ,�������к�����λ��;(5)��̯��,�þ����к���ͭԭ��8�� +6��
+6�� =4��,��ԭ��4��,������1��1�Ĺ�ϵ,��˸��Ȼ���Ļ�ѧʽΪCuCl��
=4��,��ԭ��4��,������1��1�Ĺ�ϵ,��˸��Ȼ���Ļ�ѧʽΪCuCl��
��ϰ��ϵ�д�
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
�����Ŀ

 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4�����������������������
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4�����������������������

 ��ÿ��COCl2�����ں���________���Ҽ���________���м���������ԭ�Ӳ�ȡ________�ӻ������ʽ��
��ÿ��COCl2�����ں���________���Ҽ���________���м���������ԭ�Ӳ�ȡ________�ӻ������ʽ��


 NH4++NH2���������ӻ�����Ϊl��0��l0��30���ֽ�2��3g������Ͷ��1��0 LҺ���У�����ȫ��Ӧ����NaNH2��������Һ��������䣬������Һ��NH4+��Ũ��
NH4++NH2���������ӻ�����Ϊl��0��l0��30���ֽ�2��3g������Ͷ��1��0 LҺ���У�����ȫ��Ӧ����NaNH2��������Һ��������䣬������Һ��NH4+��Ũ��