��Ŀ����
��1��Fe3+������SCN-��CN-��F-���л����ӵ��γɺܶ������
��д����̬Fe3+�ĺ�������Ų�ʽ ��
����֪(CN)2��ֱ���ͷ��ӣ����жԳ��ԣ���(CN)2�Цм��ͦҼ��ĸ�����Ϊ ��
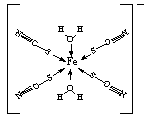
����ͼ��SCN-��Fe3+�γɵ�һ������������������е���λ�����Լ�ͷ��ʾ����
��F-��������Fe3+�γ�[FeF6]3+����������Mg2+��K+�γ�һ��������ϵ�����Ӿ��壨����ͼ�����þ���Ļ�ѧʽΪ ��


��2��������һ����Ҫ�Ļ���ԭ�ϡ�
��Һ����ˮ����,Ҳ�ܷ������룺NH3+NH3 NH4++NH2���������ӻ�����Ϊl��0��l0��30���ֽ�2��3g������Ͷ��1��0 LҺ���У�����ȫ��Ӧ����NaNH2��������Һ��������䣬������Һ��NH4+��Ũ��
NH4++NH2���������ӻ�����Ϊl��0��l0��30���ֽ�2��3g������Ͷ��1��0 LҺ���У�����ȫ��Ӧ����NaNH2��������Һ��������䣬������Һ��NH4+��Ũ��
Ϊ ��
����֪��N2(g)+O2(g)��2NO(g) ��H����180kJ��mol��l
4NH3(g)+5O2(g)��4NO(g)+6H2O(g) ��H����908 kJ��mol��l
д��������һ�������������ɵ�������̬ˮ���Ȼ�ѧ����ʽ�� ��
��3������ͼװ���У���ͨ��һ��ʱ�����װ�����缫�������ӡ�

������˵��������� ��
����Cu�缫����������2.16 g, ����Һ���Ϊ200mL, �����Һ��pH= ��
��д����̬Fe3+�ĺ�������Ų�ʽ ��
����֪(CN)2��ֱ���ͷ��ӣ����жԳ��ԣ���(CN)2�Цм��ͦҼ��ĸ�����Ϊ ��
����ͼ��SCN-��Fe3+�γɵ�һ������������������е���λ�����Լ�ͷ��ʾ����
��F-��������Fe3+�γ�[FeF6]3+����������Mg2+��K+�γ�һ��������ϵ�����Ӿ��壨����ͼ�����þ���Ļ�ѧʽΪ ��


��2��������һ����Ҫ�Ļ���ԭ�ϡ�
��Һ����ˮ����,Ҳ�ܷ������룺NH3+NH3
 NH4++NH2���������ӻ�����Ϊl��0��l0��30���ֽ�2��3g������Ͷ��1��0 LҺ���У�����ȫ��Ӧ����NaNH2��������Һ��������䣬������Һ��NH4+��Ũ��
NH4++NH2���������ӻ�����Ϊl��0��l0��30���ֽ�2��3g������Ͷ��1��0 LҺ���У�����ȫ��Ӧ����NaNH2��������Һ��������䣬������Һ��NH4+��Ũ��Ϊ ��
����֪��N2(g)+O2(g)��2NO(g) ��H����180kJ��mol��l
4NH3(g)+5O2(g)��4NO(g)+6H2O(g) ��H����908 kJ��mol��l
д��������һ�������������ɵ�������̬ˮ���Ȼ�ѧ����ʽ�� ��
��3������ͼװ���У���ͨ��һ��ʱ�����װ�����缫�������ӡ�

������˵��������� ��
| A���������缫��Ӧʽ��Cu2++2e-��Cu |
| B�����һ��ʱ���װ�ñ���pH��С |
| C�������ͨ��������HCl���壬��ʹ��Һ�ָ������ǰ��״̬ |
| D�����һ��ʱ��������м���0.1molCu(OH)2��ʹ�������Һ��ԭ,���·��ͨ���ĵ���Ϊ0.2mol������������Һ����MgCl2,�����ܷ�Ӧ�����ӷ���ʽΪ�� |
��16�֣���1����1s22s22p63s33p63d5�� [Ar]3d5 ��2�֣� �� 4��3 ��1�֣�
�� ��1�֣� ��KMgF3��2������������Ҳ�ɸ��֣�
��1�֣� ��KMgF3��2������������Ҳ�ɸ��֣�
��2����10��29 mol��L��1��2�֣� ��4NH3(g)+6NO(g)��5N2(g)+6H2O(g) ��H����1808kJ��mol��1��2�֣�
��3����BD ��2�֣��д���Ϊ0�֣���һ����1�֣�
��Mg2++2Cl-+2H2O Mg(OH)2��+H2��+Cl2�� ��2�֣���д������1�֣� ��13��2�֣�
Mg(OH)2��+H2��+Cl2�� ��2�֣���д������1�֣� ��13��2�֣�
��
 ��1�֣� ��KMgF3��2������������Ҳ�ɸ��֣�
��1�֣� ��KMgF3��2������������Ҳ�ɸ��֣���2����10��29 mol��L��1��2�֣� ��4NH3(g)+6NO(g)��5N2(g)+6H2O(g) ��H����1808kJ��mol��1��2�֣�
��3����BD ��2�֣��д���Ϊ0�֣���һ����1�֣�
��Mg2++2Cl-+2H2O
 Mg(OH)2��+H2��+Cl2�� ��2�֣���д������1�֣� ��13��2�֣�
Mg(OH)2��+H2��+Cl2�� ��2�֣���д������1�֣� ��13��2�֣������������1��������ԭ��������26������ݹ���ԭ����֪��̬Fe3+�ĺ�������Ų�ʽΪ1s22s22p63s33p63d5�� [Ar]3d5��
����֪(CN)2��ֱ���ͷ��ӣ����жԳ��ԣ���˸û�����ĽṹʽӦ����C��N��C��N�����ڵ������ǦҼ�����������1���Ҽ���2���м����ɵģ�����(CN)2�Цм��ͦҼ��ĸ�����Ϊ4��3��
��SCN-��Fe3+�γɵ������������ԭ�Ӻ��пչ����S��Oԭ�Ӻ��й¶Ե��ӣ��Ӷ��γ���λ�������������е���λ�����Լ�ͷ��ʾ��Ϊ
 ��
���ܸ��ݾ����ṹ
 ��֪��þ�����ڶ��㴦�������������ϡ������������Ĵ������Ը��ݾ�̯����֪�������к��е�Mg2����F����K�������ֱ���8��
��֪��þ�����ڶ��㴦�������������ϡ������������Ĵ������Ը��ݾ�̯����֪�������к��е�Mg2����F����K�������ֱ���8��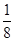 ��1��12��
��1��12��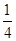 ��3��1�����Ը����ʵĻ�ѧʽ��KMgF3��
��3��1�����Ը����ʵĻ�ѧʽ��KMgF3����2����2.3g�Ƶ����ʵ�����2.3g��23g/mol��0.1mol�������ԭ���غ��֪NaNH2�����ʵ���Ҳ��0.1mol�����NH2����Ũ����0.1mol/L�����Ը������ӻ�����Ϊl��0��l0��30��֪����Һ��NH4+��Ũ�ȣ�
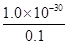 ��1��10��29 mol/L��
��1��10��29 mol/L������֪��I��N2(g)+O2(g)��2NO(g) ��H��180kJ��mol��l����4NH3(g)+5O2(g)��4NO(g)+6H2O(g) ��H����908 kJ��mol��l������ݸ�˹���ɿ�֪����I��5���õ���Ӧ4NH3(g)+6NO(g)��5N2(g)+6H2O(g)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H����908 kJ��mol��l��180kJ��mol��l��5����1808kJ��mol��1�����Ȼ�ѧ����ʽΪ4NH3(g)+6NO(g)��5N2(g)+6H2O(g) ��H����1808kJ��mol��1��
д��������һ�������������ɵ�������̬ˮ���Ȼ�ѧ����ʽ
��3����A��ͨ��һ��ʱ�����װ�����缫�������ӣ���˵���õ缫����������Һ�е�������ͭ���ӷŵ�����ͭ���缫��Ӧʽ��Cu2++2e-��Cu��A��ȷ��B��X�ǵ�Դ�ĸ�����Y�����������װ�������缫��������ͭ�缫�����������Ը�װ���ǵ�Ƴأ�����ͭ�϶�������Һ��pH���䣬B����ȷ��C����װ���ǵ���Ȼ�����Һ�����������������ء����������������������ͨ��������HCl���壬��ʹ��Һ�ָ������ǰ��״̬��C��ȷ��D�����һ��ʱ��������м���0.1molCu(OH)2��ʹ�������Һ��ԭ,�������ԭ���غ��֪��������������������ʵ�����0.1mol�����Ե�·��ͨ���ĵ���Ϊ0.1mol��4��0.4mol��D����ȷ����ѡBD��
������������Һ����MgCl2,�������������ӷŵ磬�����������ӷŵ磬�����ﻹ��������þ��ɫ�����������ܷ�Ӧ�����ӷ���ʽΪMg2++2Cl-+2H2O
 Mg(OH)2��+H2��+Cl2����
Mg(OH)2��+H2��+Cl2��������Cu�缫����������2.16 g, ����������������2.16g�����ʵ�����2.16g��108g/mol��0.02mol�����ݵ��ӵ�ʧ�غ��֪����װ��ҲӦ��ת��0.02mol���ӣ����Է�Ӧ������0.02mol�������ƣ��������Ƶ����ʵ���Ũ�ȣ�0.02mol��0.2L��0.1mol/L��������Һ��pH��13��

��ϰ��ϵ�д�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
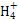 ���幹���� ,������ԭ�ӵ��ӻ���ʽΪ ��
���幹���� ,������ԭ�ӵ��ӻ���ʽΪ ��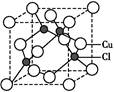


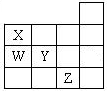


 ������Ŀ֮��Ϊ ��
������Ŀ֮��Ϊ ��
