��Ŀ����
�����������С�⣺��1����ѧʵ�����ע�ⰲȫ�������������ڰ�ȫ��������___________��ѡ����ĸ����
A.������ԭ����ͭʵ���У��ȼ�������ͭ��ͨ����
B.����ʯ��ʱ������һ��ʱ�����δ�����Ƭ�����̰ο���Ƥ����Ͷ�����Ƭ
C.ʵ�������Ƶ�ʵ��ʱ�����µ���мͶ�뵽��Һ����
D.����Ũ������ƾ����Һʱ����1����ľƾ�����3�����Ũ������
E.Ƥ����մ������Ũ����ʱ�������ô���ˮ��ϴ����Ϳ��ϡ̼��������Һ
F.����ϩʱ��������Ϊ300 ����¶ȼƴ�������Ϊ200 ����¶ȼƣ��ⷴӦҺ���¶�
��2����ӵ������ⶨ������ͭ������ԭ���ͷ������£�
��֪���������������£�������Cu2+��I-���ö�������I2��I2���ڹ�����KI��Һ�У�
I2+I-====![]() ����֪�����ԣ�Fe3+��Cu2+��I2��
����֪�����ԣ�Fe3+��Cu2+��I2��![]() ��
��
������I2����c mol��L-1 Na2S2O3����Һ�ζ���2![]() +
+![]() ====
====![]() +3I-��
+3I-��
ȷ��ȡa g��������������250 mL����ƿ����ĥ��������ƿ���У���50 mL����ˮ��5 mL 3 mol��L-1 H2SO4��Һ��������NaF���ټ���������10% KI��Һ��ҡ�ȡ����ϵ���ƿƿ�ǣ����ڰ���5 min����ַ�Ӧ����1��2 mL 05%�ĵ�����Һ����Na2S2O3����Һ�ζ�����ɫ��ȥʱ������ȥV mL��Һ��
��ʵ���У��ڼ�KIǰ���������NaF���Ʋ������ÿ����ǣ�______________________��
��ʵ���м���5 mL 3 mol��L-1 H2SO4��Һ������Ϊ����������ǣ�_______________________��
�۱�ʵ�����õ���ƿ��������ͨ��ƿ����Ϊ��_____________________________________��
������ͭ��Һ��⻯����Һ��Ӧ���ɰ�ɫ�������⻯��ͭ���������⣬�÷�Ӧ�����ӷ���ʽΪ��____________________________________________________________________��
�ݸ��ݱ���ʵ�������õ���������ͭԪ�ص���������Ϊw(Cu)=______________��
��1��ABCD
��2�����ڱ�Fe3+����ֹ���ƫ������ ���ṩ�����Ի�����������ֹͭ����ˮ�� �۷�ֹ�����е�������⻯�ط�Ӧ����ֹ���ɵĵ����� ��2Cu2++4I-====2CuI��+I2 ��![]() ��100%
��100%

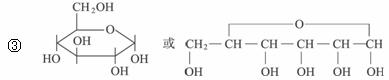
 д������A�Ļ�ѧ����ʽ��_________________________________��
д������A�Ļ�ѧ����ʽ��_________________________________��