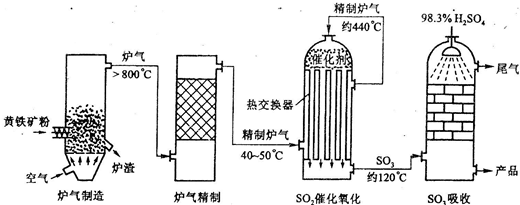
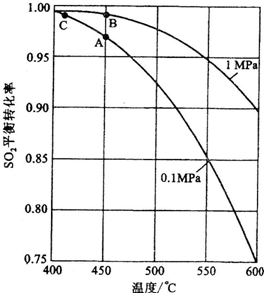
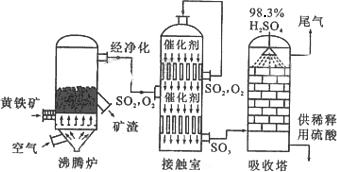
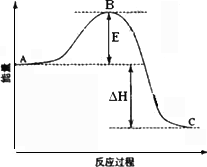
��Ŀ����
�Ի�����Ϊԭ���������ᣬ�����������������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2����Ρ�
(1)SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����
Br2�����ӷ���ʽ��____________________��
(2)Ϊ�ⶨ������е�Ԫ�ص���������������ͬ��������ηֱ����100. 00 mL��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ������������ֲⶨ������±���
(1)SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����
Br2�����ӷ���ʽ��____________________��
(2)Ϊ�ⶨ������е�Ԫ�ص���������������ͬ��������ηֱ����100. 00 mL��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ������������ֲⶨ������±���
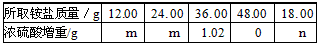
���㣨Ҫ��д�����̣���
������NaOH��Һ�����ʵ���Ũ�ȡ�
�ڸ�����е�Ԫ�ص����������Ƕ��٣�
������NaOH��Һ�����ʵ���Ũ�ȡ�
�ڸ�����е�Ԫ�ص����������Ƕ��٣�
(1)SO2+Br2+2H2O=4H++2Br-+SO42-
(2)����12.00 g��24.00 gʱŨ�������ӵ�������ͬ��˵���������ʽ�δ��ڣ����������Ƶ����ʵ���Ũ��Ϊc mol/L��12.00 g�������x mol��(NH4)2SO4��ymol��NH4HSO4����132x+115y =12.00 ��
12.00 gʱ��β��㣬��N�غ�֪n(NH3) =2x +y��24. 00 gʱ����Ѿ�������(NH4)2SO4Ϊ2x mol��
NH4HSO4Ϊ2y mol�������������Ⱥ�HSO4-��Ӧ��
12.00 gʱ��β��㣬��N�غ�֪n(NH3) =2x +y��24. 00 gʱ����Ѿ�������(NH4)2SO4Ϊ2x mol��
NH4HSO4Ϊ2y mol�������������Ⱥ�HSO4-��Ӧ��
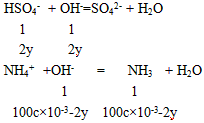
����12.00 g��24.00 g��β����İ���һ���࣬n(NH3)=2x+y=100c��l0-3-2y ��
36.00 gʱ��ι�����(NH4)2SO4Ϊ 3x mol, NH4HSO4Ϊ3y mol,��n(NH3) =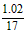 mol = 0. 06 mol ��
mol = 0. 06 mol ��
36.00 gʱ��ι�����(NH4)2SO4Ϊ 3x mol, NH4HSO4Ϊ3y mol,��n(NH3) =
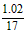 mol = 0. 06 mol ��
mol = 0. 06 mol ��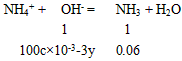
����100c��10-3-3y =0.06 ��
�����٢ڢ۽��x=0.03,y=0.07,c=2.7��
�ڵ��������ٷֺ���=(2x+y)��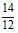 ��100% =(0.06+0. 07)��
��100% =(0.06+0. 07)��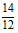 ��100% = 15.17% ��
��100% = 15.17% ��
�����٢ڢ۽��x=0.03,y=0.07,c=2.7��
�ڵ��������ٷֺ���=(2x+y)��
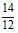 ��100% =(0.06+0. 07)��
��100% =(0.06+0. 07)��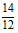 ��100% = 15.17% ��
��100% = 15.17% �� 
��ϰ��ϵ�д�
�����Ŀ