��Ŀ����
10�����б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ���ǣ�������| A�� | ��������ʯ��ˮ����С�մ���Һ��HCO${\;}_{3}^{-}$+OH-+Ca2+=CaCO3��+H2O | |
| B�� | ������Һ�м�������������Һ��H++SO${\;}_{4}^{2-}$+Ba2++OH-�TBaSO4��+H2O | |
| C�� | ̼���Ͷ�뵽���CO${\;}_{3}^{2-}$+2H+�TCO2��+H2O | |
| D�� | ��Ƭ���뵽ϡ������Һ�У�Fe+2H+�TFe3++H2�� |
���� A����С�մ���Һ�м�������ij���ʯ��ˮ��С�մ���ȫ��Ӧ������̼��ơ�NaOH��ˮ��
B�����Ӹ�����Ȳ��������ʽṹ��
C��̼���Ϊ������Ӧ������ѧʽ��
D����ɲ��غ㣮
��� �⣺A����С�մ���Һ�м�������ij���ʯ��ˮ�����ӷ�ӦΪHCO3-+Ca2++OH-�TCaCO3��+H2O����A��ȷ��
B��������Һ�м�������������Һ�����ӷ���ʽ��2H++SO${\;}_{4}^{2-}$+Ba2++2OH-�TBaSO4��+2H2O����B����
C��̼���Ͷ�뵽���ᣬ���ӷ���ʽ��CaCO3+2H+�TCO2��+H2O+Ca2+����C����
D����Ƭ���뵽ϡ������Һ�У����ӷ���ʽ��Fe+2H+�TFe2++H2������D����
��ѡ��A��
���� ���⿼�����ӷ�Ӧ����ʽ��д��Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ӷ�Ӧ����д����Ϊ���Ĺؼ���ע�⻯ѧʽ�IJ�֣���Ӧ�������Է�Ӧ��Ӱ�죬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
20�����и��������У���������ͬ���ǣ�������
| A�� | 2L SO2��2L CO2 | B�� | 9gˮ�ͱ�״����11.2L CO2 | ||
| C�� | ��״����1mol������22.4Lˮ | D�� | 0.2mol H2S��2.24L HCl |
1�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã����ٵ����������ڴ����е��ŷ��ǻ�����������Ҫ����֮һ��
��1����ͼ1��1mol NO2�����1mol CO���巴Ӧ����CO2�����NO��������������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��NO2��g��+CO��g���TCO2��g��+NO��g����H=-234kJ•mol-1��
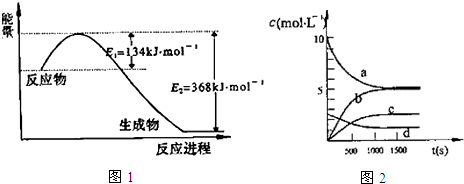
��֪��N2��g��+2NO2��g��?4NO��g����H=+292.3kJ•mol-1��
��Ӧ��2NO��g��+2CO��g��?N2��g��+2CO2��g���ġ�H=-760.3kJ•mol-1��
��2��һ���¶��£������Ϊ2L�ĺ����ܱ������г���20mol NO2��5mol O2������Ӧ��4NO2��g��+O2��g��?2N2O5��g������֪��ϵ��n��NO2����ʱ��仯�����
��д���÷�Ӧ��ƽ�ⳣ������ʽ��K=$\frac{{c}^{2}��{N}_{2}{O}_{5}��}{{c}^{4}��N{O}_{2}����c��{O}_{2}��}$��
��֪��K3000C��K3500C����÷�Ӧ�Ƿ��ȷ�Ӧ������ȡ������ȡ�����
�ڷ�Ӧ�ﵽƽ���NO2��ת����Ϊ49.6%����Ҫ����NO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��AD
A�������¶� B�����뺤����ʹ��ϵѹǿ���� C���ٳ���NO2 D���ٳ���4mol NO2��1mol O2
��ͼ2�б�ʾN2O5��Ũ�ȵı仯������c����O2��ʾ��0��500s�ڸ÷�Ӧ��ƽ������v=0.00151 mol•L-1•s-1��
��1����ͼ1��1mol NO2�����1mol CO���巴Ӧ����CO2�����NO��������������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��NO2��g��+CO��g���TCO2��g��+NO��g����H=-234kJ•mol-1��

��֪��N2��g��+2NO2��g��?4NO��g����H=+292.3kJ•mol-1��
��Ӧ��2NO��g��+2CO��g��?N2��g��+2CO2��g���ġ�H=-760.3kJ•mol-1��
��2��һ���¶��£������Ϊ2L�ĺ����ܱ������г���20mol NO2��5mol O2������Ӧ��4NO2��g��+O2��g��?2N2O5��g������֪��ϵ��n��NO2����ʱ��仯�����
| t��s�� | 0 | 500 | 1000 | 1500 |
| n��NO2����mol�� | 20 | 13.96 | 10.08 | 10.08 |
��֪��K3000C��K3500C����÷�Ӧ�Ƿ��ȷ�Ӧ������ȡ������ȡ�����
�ڷ�Ӧ�ﵽƽ���NO2��ת����Ϊ49.6%����Ҫ����NO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��AD
A�������¶� B�����뺤����ʹ��ϵѹǿ���� C���ٳ���NO2 D���ٳ���4mol NO2��1mol O2
��ͼ2�б�ʾN2O5��Ũ�ȵı仯������c����O2��ʾ��0��500s�ڸ÷�Ӧ��ƽ������v=0.00151 mol•L-1•s-1��
5��ij��Һ�к���SO32-��SiO32-��Br -CO32-��Na+�������Һ��ͨ�������Cl2�������ж���ȷ���ǣ�������
�ٷ�Ӧǰ����Һ������Ũ�Ȼ������ֲ������Na+ ���н�״��������
����������� ����Һ��ɫ�����仯 ����Һ�й�������2��������ԭ��Ӧ��
�ٷ�Ӧǰ����Һ������Ũ�Ȼ������ֲ������Na+ ���н�״��������
����������� ����Һ��ɫ�����仯 ����Һ�й�������2��������ԭ��Ӧ��
| A�� | �٢ڢۢ� | B�� | �٢ڢۢܢ� | C�� | �٢ۢܢ� | D�� | �ڢܢ� |
15�����������ڵ���ʵ�һ���ǣ�������
| A�� | ʯī��������Һ��ʳ�ξ��� | B�� | ����״̬��KOH����̬��NaCl | ||
| C�� | ϡH2SO4��NaOH��Һ����HNO3 | D�� | Һ����ʯ��ˮ��ˮ�� |
2�����ձ��м���ˮ�ͱ��������ܶ�Ϊ0.88g/cm3����ˮ�������ܣ��Ҳ����Ʒ�Ӧ����50mL����һС�������ƣ��ܶ�Ϊ0.97g/cm3��Ͷ���ձ��У��۲쵽���������Ϊ��������
| A�� | ���ڱ���ˮ�Ľ��洦��Ӧ�������ϸ��³� | |
| B�� | ��ͣ���ڱ����в�������Ӧ | |
| C�� | ���ڱ���Һ���Ϸ�Ӧ���Ĵ��ζ� | |
| D�� | ����ˮ���з�Ӧ���Ĵ��ζ� |
20����֪KClO3+6HCl�TKCl+3Cl2��+3H2O�����ڴ˷�Ӧ��˵����ȷ���ǣ�������
| A�� | KClO3����ԭ������ԭ�� | |
| B�� | �������뻹ԭ�������ʵ���֮��Ϊ1��6 | |
| C�� | ���������뻹ԭ�����������Ϊ5��1 | |
| D�� | ������HCl��Cl2��KClO3 |
 ��
��