��Ŀ����
����Ŀ��������Ԫ��A��B��C��D��AԪ�ص�ԭ���������������ڲ��������������BΪ�ؿ��к�������Ԫ�أ�C��ԭ�Ӱ뾶���Ķ���������Ԫ�أ�C��D�γɵ����ӻ�����CD�dz��õĵ�ζƷ����д���пհף�
��1��A B2�Ľṹʽ_______________��CԪ�������ڱ��е�λ�õ�_______����________��
��2��B��C��ɵ�һ�ֻ�������ˮ�������Ϸ�Ӧ�Ļ�ѧ����ʽΪ��____________________
��3����ͼ��ʾ�������a��ҺΪ����CD�ı�����Һ��XΪʯī�缫��YΪ���缫����ֱͨ����Դ��
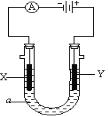
X�缫�ĵ缫��ӦʽΪ________________________��
Y�缫�ĵ缫��ӦʽΪ________________________��
��4�������£���ͬ�����0.2mol��L��1CD��Һ��0.1mol��L��1 C2AB3��Һ�У���������Ŀ�϶����______________��Һ���ѧʽ����
���𰸡�O=C=O 3 IA Na2O + H2O == 2NaOH 2H+ + 2e�� = H2������2H2O + 2e�� = H2��+ 2OH���� Fe��2e��= Fe2+ NaCl
��������
������Ԫ��A��B��C��D��AԪ�ص�ԭ���������������ڲ��������������AΪCԪ�أ�BΪ�ؿ��к�������Ԫ�أ�BΪOԪ�أ�C��ԭ�Ӱ뾶���Ķ���������Ԫ�أ�CΪ�ƣ�C��D�γɵ����ӻ�����NaCl�dz��õĵ�ζƷ��
������Ԫ��A��B��C��D�ֱ�Ϊ��C��O��Na��Cl��
��1��������̼�е�̼�����4�����ӣ��ֱ���O�γ��������ۼ���AB2�ĽṹʽO=C=O��CԪ��Ϊ��Ԫ�أ������ڱ���λ�ڵ�3����IA�壻
��2��B��C��ɵ�һ�ֻ������������ƣ���ˮ�������Ϸ�Ӧ�Ļ�ѧ����ʽΪ��Na2O + H2O == 2NaOH��
��3�����NaCl�ı�����Һ��XΪʯī�缫�����Դ������������������X�缫�ĵ缫��ӦʽΪ 2H+ + 2e�� = H2������2H2O + 2e�� = H2��+ 2OH������YΪ���缫����ֱ����Դ��������������������Ӧ��Y�缫�ĵ缫��ӦʽΪFe��2e��= Fe2+��
��4�������£���ͬ�����0.2mol��L��1NaCl��Һ��0.1mol��L��1Na2CO3��Һ�У�����������ͬ��̼�������ˮ�⣬�������������������ӣ���������Ŀ�϶����NaCl��Һ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����ס��ҡ��������ܱ������г���һ������A��B��������Ӧ��A(g)+xB(g) ![]() 2C (g)���������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ����±�����ͼ��ʾ��
2C (g)���������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ����±�����ͼ��ʾ��
���� | �� | �� | �� |
�ݻ� | 0.5L | 0.5L | 1.0L |
�¶�/�� | T1 | T2 | T2 |
��Ӧ�� ��ʼ�� | 1.5molA 0.5molB | 1.5molA 0.5molB | 6.0molA 2.0molB |

����˵����ȷ����
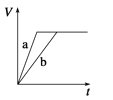
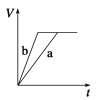
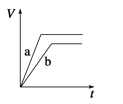
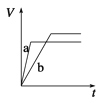
A. 10min�ڼ������з�Ӧ��ƽ������v(A)=0.025mol/(Lmin)
B. ��ͼ��֪��T1��T2���Ҹ÷�ӦΪ���ȷ�Ӧ
C. x=1����ƽ��ʱ�����¶Ȳ��䣬�ı��������ƽ���ƶ�
D. T1������ʼʱ�������г���0.5molA��1.5molB��ƽ��ʱA��ת����Ϊ75%