��Ŀ����
�������и��������������ݣ��ɷֱ�����䡰���ʵ����������������ʵ����ʵ���Ũ�ȡ������жϲ���⡣
��1����NA��ʾ�����ӵ���������ֵ����ij����������ҺV L�к���N��OH-������������Һ��______Ϊ______��
��2����֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a������������Һ��______Ϊ______��
��3����֪��״����1���ˮ���ܽ�500������Ȼ��⣬��������״�����Ȼ��ⱥ����Һ��______Ϊ______��
��4����֪��100 mL�Ȼ�����ˮ��Һ�����������գ��ɵõ���ɫ����b g��������ԭ�Ȼ�����Һ��______Ϊ______��
��1����NA��ʾ�����ӵ���������ֵ����ij����������ҺV L�к���N��OH-������������Һ��______Ϊ______��
��2����֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a������������Һ��______Ϊ______��
��3����֪��״����1���ˮ���ܽ�500������Ȼ��⣬��������״�����Ȼ��ⱥ����Һ��______Ϊ______��
��4����֪��100 mL�Ȼ�����ˮ��Һ�����������գ��ɵõ���ɫ����b g��������ԭ�Ȼ�����Һ��______Ϊ______��
��1��NaOH�����ʵ���Ũ�� 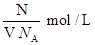
��2��NaOH����������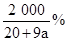
��3��HCl���������� 44.9%
��4��AlCl3�����ʵ���Ũ��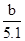 mol/L
mol/L
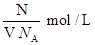
��2��NaOH����������
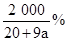
��3��HCl���������� 44.9%
��4��AlCl3�����ʵ���Ũ��
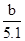 mol/L
mol/L��1����֪��Һ���ܶȣ�ֻ��������ʵ���Ũ�ȣ�c��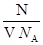 mol/L��
mol/L��
��2��NaOH��H2O�����ʵ���֮��Ϊ1��a���������ʵ�����������w��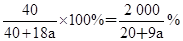 ��
��
��3����֪��Һ���ܶȣ����ܼ������ʵ���Ũ�ȣ�����������������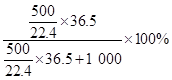
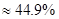 ��
��
��4����ɫ����ΪAl2O3��n��Al2O3����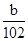 mol��n��AlCl3����
mol��n��AlCl3����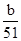 mol��c��AlCl3����
mol��c��AlCl3����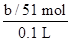 ��
��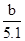 mol/L��
mol/L��
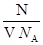 mol/L��
mol/L����2��NaOH��H2O�����ʵ���֮��Ϊ1��a���������ʵ�����������w��
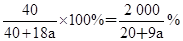 ��
����3����֪��Һ���ܶȣ����ܼ������ʵ���Ũ�ȣ�����������������
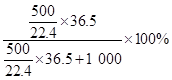
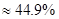 ��
����4����ɫ����ΪAl2O3��n��Al2O3����
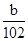 mol��n��AlCl3����
mol��n��AlCl3����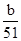 mol��c��AlCl3����
mol��c��AlCl3����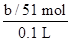 ��
��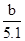 mol/L��
mol/L��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2MO+4NO2��+O2��������29.6g M��NO3��2ʹ����ȫ�ֽ⣬�ڱ�״�����ռ�11.2L�����壬��ôM��Ħ��������
2MO+4NO2��+O2��������29.6g M��NO3��2ʹ����ȫ�ֽ⣬�ڱ�״�����ռ�11.2L�����壬��ôM��Ħ��������