��Ŀ����
��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӣ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӣ�������Ԫ��W�ĵ����ǵ���ɫ��ĩ״���壬�ڿ�����ȼ�����ɵ��������γ��������Ҫ��Ⱦ��֮һ��Ԫ��R�ĵ����ڳ��³�ѹ���ǻ���ɫ���壬������ˮ����ش��������⣺
��1��XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ
��2��YԪ��ԭ�ӵļ۵��ӵĹ����ʾʽΪ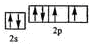
 ����Ԫ�ص�������
����Ԫ�ص�������
��3��X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ
��4����֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��
��5��W��R ��Ԫ�طǽ����Խ�ǿ����
��1��XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ
1s22s22p63s23p63d104s24p3
1s22s22p63s23p63d104s24p3
����Ԫ�صķ�����As
As
����2��YԪ��ԭ�ӵļ۵��ӵĹ����ʾʽΪ


��
��
����3��X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ
����
����
����4����֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��
As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O
As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O
����5��W��R ��Ԫ�طǽ����Խ�ǿ����
Cl
Cl
��дԪ�ط��ţ���д���ܹ�֤����һ���۵�һ��ʵ����ʵ�����������ǿ��������Ȼ�����ȶ���ǿ������
�����������ǿ��������Ȼ�����ȶ���ǿ������
����������1�����ݻ�̬ԭ�Ӻ�������Ų�ʽ����д�����ͺ��������=ԭ���������ش�
��2������Ԫ��ԭ�ӵļ۵��ӹ���Ų�ʽ���ش�
��3�����ݰ������ӽṹ���ƶϣ�
��4�����ݷ�Ӧ������������غ��ϵ����д����ʽ��
��5��ͬ���ڴ����ҷǽ���������ǿ�����ݷǽ�����ǿ�����жϷ������ش�
��2������Ԫ��ԭ�ӵļ۵��ӹ���Ų�ʽ���ش�
��3�����ݰ������ӽṹ���ƶϣ�
��4�����ݷ�Ӧ������������غ��ϵ����д����ʽ��
��5��ͬ���ڴ����ҷǽ���������ǿ�����ݷǽ�����ǿ�����жϷ������ش�
����⣺��1��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ������֪��XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ1s22s22p63s23p63d104s24p3�����������=ԭ������=33����ΪAs���ʴ�Ϊ��1s22s22p63s23p63d104s24p3��As��
��2��YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӣ���Ԫ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ1s22s22p4��Ϊ��ԭ�ӣ���۵��ӵĹ����ʾʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ������
������
��3��X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��Xԭ������Ϊ33��YΪ8����ZΪHԭ�ӣ�X��Z���γɻ�����AsH3���û�����Ŀռ乹�ͺͰ������ƣ�Ϊ�����ͣ��ʴ�Ϊ��������
��4����֪������As2O3��ϡ������Һ�пɱ�����п��ԭΪAsH3�����ﻹ��ZnSO4��H2O��
��Ӧ����ʽΪ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O���ʴ�Ϊ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O��
��5������Ԫ��W�ĵ����ǵ���ɫ��ĩ״���壬�ڿ�����ȼ�����ɵ��������γ��������Ҫ��Ⱦ��֮һ������ȷ��W��S��Ԫ��R�ĵ����ڳ��³�ѹ���ǻ���ɫ���壬������ˮ������ȷ��R��Cl��Cl�ķǽ�����ǿ��S����Ϊ�⻯��Խ�ȶ���Ԫ�طǽ�����Խǿ������������Ӧˮ��������Խǿ����Ԫ�طǽ�����Խǿ��
�ʴ�Ϊ��Cl�������������ǿ�����ᣨ���Ȼ�����ȶ���ǿ�����⣩��
��2��YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӣ���Ԫ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ1s22s22p4��Ϊ��ԭ�ӣ���۵��ӵĹ����ʾʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ������
������ ��3��X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��Xԭ������Ϊ33��YΪ8����ZΪHԭ�ӣ�X��Z���γɻ�����AsH3���û�����Ŀռ乹�ͺͰ������ƣ�Ϊ�����ͣ��ʴ�Ϊ��������
��4����֪������As2O3��ϡ������Һ�пɱ�����п��ԭΪAsH3�����ﻹ��ZnSO4��H2O��
��Ӧ����ʽΪ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O���ʴ�Ϊ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O��
��5������Ԫ��W�ĵ����ǵ���ɫ��ĩ״���壬�ڿ�����ȼ�����ɵ��������γ��������Ҫ��Ⱦ��֮һ������ȷ��W��S��Ԫ��R�ĵ����ڳ��³�ѹ���ǻ���ɫ���壬������ˮ������ȷ��R��Cl��Cl�ķǽ�����ǿ��S����Ϊ�⻯��Խ�ȶ���Ԫ�طǽ�����Խǿ������������Ӧˮ��������Խǿ����Ԫ�طǽ�����Խǿ��
�ʴ�Ϊ��Cl�������������ǿ�����ᣨ���Ȼ�����ȶ���ǿ�����⣩��
���������⿼��ѧ��ԭ�ӵ���ɺͽṹ֪ʶ�Լ�Ԫ�����ڱ���Ԫ�������ɵ�Ӧ��֪ʶ�����Ը��ݽ̲�֪ʶ���ش��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ