��Ŀ����
��15�֣��±���ʵ�����Ʊ�������й����ݣ�
|
��� |
ʵ������ |
ʵ��ԭ�� |
����װ�� |
|
�� |
������ |
|
|
|
�� |
�ư��� |
|
|
|
�� |
������ |
|
|
(1)���ݱ�������ʵ��ԭ����������װ����ѡ����ʵķ���װ�ã������������ϱ��Ŀո��С�

(2)���������У��ӷ�Ӧ��������ת�ƵĽǶȿ������Բ�ͬ������������� ��д��ʵ������ȡ������Ļ�ѧ����ʽ .
��3�����������Ʊ�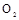 ��װ���Ʊ�
��װ���Ʊ�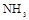 ,Ӧѡ����Լ�Ϊ
.
,Ӧѡ����Լ�Ϊ
.
(4)�Ʊ� ����
���� ������1OOml,����
������1OOml,���� �����������ơ�
�����������ơ�
����Ҫ����Ͳ��ȡ ����������Ϊ mL��
����������Ϊ mL��
��ʵ�����ṩ������������Ϊ���������Ҫѡ�������Ϊ(�����) ��
A.100mL��Ͳ B.������ƽ C.������ D.50mL����ƿ
E.lOmL��Ͳ F.��ͷ�ι� G.lOOmL�ձ� H.lOOmL����ƿ
������ʵ������У�����ȷ���� (��д���)��
A.ʹ������ƿǰ��������Ƿ�©ˮ
B.����ƿ������ˮϴ�������ô�����Һ��ϴ
C.������Һʱ������Ͳ��ȡŨ���Ტ�ز�������������ƿ�У�������������ˮ������̶���1��2cm�������ý�ͷ�ιܵμ�����ˮֱ����Һ�����͵�Ϳ̶�����ƽ
D.���ݺ�Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ��ת��ҡ�����
����15�֣���1����ÿ��1�֣���3�֣�
|
��� |
����װ�� |
|
�� |
C |
|
�� |
B |
|
�� |
A��C |
��2��NH3(1��)
Ca(OH)2+2NH4Cl CaCl2+2NH3��+2H2O(2��)
CaCl2+2NH3��+2H2O(2��)
��3��Ũ��ˮ���������ƣ�����ʯ�ҵȣ���2�֣�
��4����66.7��2�֣���ACFGH��2�� ����BC(2��)
����������1�����鳣��������Ʊ���˫��ˮ�ֽⲻ��Ҫ���ȣ����ѡ��Cװ�ã�ʵ������ȡ���������ù���֮��ķ�Ӧ���ҷ�Ӧ��Ҫ���ȣ�����ѡ��Bװ�ã�ʵ������ȡ���������ù������Һ��֮��ķ�Ӧ���ҷ�Ӧ��Ҫ���ȣ�ѡ��Aװ�ã�����ø��������Һ����Ũ���ᣬ����Ҫ���ȣ����Ҳ����ѡ��Cװ�á�
��2��ʵ������ȡ�����Ƿ�������ԭ��Ӧ������ȡ�����������ķ�Ӧ����������ԭ��ʵ������ȡ�����ķ���ʽ��Ca(OH)2+2NH4Cl CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��3���������Ҫ������ȡ��������������ð�ˮ�IJ��ȶ��ԣ��ֽ����ɰ�����ʵ�֣������Խ�Ũ��ˮ������ʯ�һ��������ƹ��塣
��4������������ʵ�����0.1L��8mol/L��0.8mol��������ҪŨ����������0.8mol��12molL��0.0667L��66.7ml��
����ȡŨ������Ҫ100ml��Ͳ���ܽ���Ҫ�ձ��Ͳ�������ת����Ҫ100ml����ƿ��������Ҫ��ͷ�ιܣ����Դ�ѡACFGH��
������ƿ������ϴ��ѡ��B����ȷ������ƿ��������ϡ�ͣ�ѡ��C����ȷ���������ȷ�ģ���ѡBC��

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
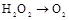
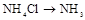
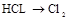



 I���±���ʵ�����Ʊ�������й����ݣ�
I���±���ʵ�����Ʊ�������й����ݣ�



