��Ŀ����
����Ŀ�������Ļ��ŵ�������ĵҶ�˹�����¶���Ӧ�����ɹ���˫ϩ��ϩ����Ȳ����Ӧ������Ԫ���ķ�Ӧ���磺
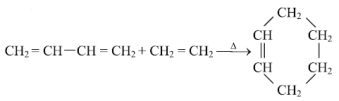 ���ɱ�ʾΪ
���ɱ�ʾΪ![]() ����ʵ���Ǹ��ӳɷ�Ӧ��
����ʵ���Ǹ��ӳɷ�Ӧ��
��ҵ�ϳ�������������ȡ��Ҫ��ѧԭ��![]() ��M����
��M����
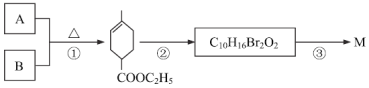
�����е�AΪ��Ȼ�ĵ��壬����ʽΪC5H8��
��1��A��������______��B�Ľṹ��ʽ��______��
��2��A������Ȳ����ͬ���칹��һ����____�֣�A��B��Ӧ���ɵ���һ�ֺ���Ԫ���Ļ�����Ľṹ��ʽΪ________��
��3���ڷ�Ӧ������Ϊ_____���۷�Ӧ�Ļ�ѧ����ʽΪ��________��
��4��������Ҫ��д��B������ͬ���칹��Ľṹ��ʽ��__________��
�����ܷ���������Ӧ�������ӽṹ�к����������������ܷ���ˮ�ⷴӦ
��5���ɱ�ϩ�Ƶ�B���������̣�
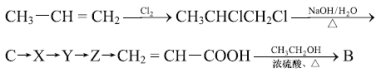
���������е�ǰ���������һ������д����д�������м䲽������̣�________��
���𰸡�2������1��3������ϩ CH2=CH-COOCH2CH3 3  �ӳɷ�Ӧ
�ӳɷ�Ӧ 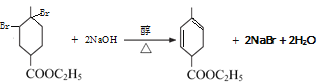 HCOO-C(CH3)=CHCH3 ��HCOO-CH=CH(CH3)2
HCOO-C(CH3)=CHCH3 ��HCOO-CH=CH(CH3)2 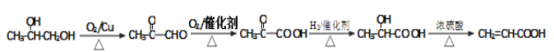
��������
AΪ��Ȼ�ĵ��壬����ʽΪC5H8����A�Ľṹ��ʽΪCH2=C(CH3)-CH=CH2������Ϊ 2������1��3������ϩ��A��B������Ϣ�еķ�Ӧ����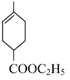 �����Ƴ�B�Ľṹ��ʽΪCH2=CH-COOC2H5�����M�Ľṹ��֪����Ӧ��Ϊ̼̼˫���ļӳɷ�Ӧ����Ӧ��Ϊ��ȥ±�������ȥ��Ӧ���Դ˷������
�����Ƴ�B�Ľṹ��ʽΪCH2=CH-COOC2H5�����M�Ľṹ��֪����Ӧ��Ϊ̼̼˫���ļӳɷ�Ӧ����Ӧ��Ϊ��ȥ±�������ȥ��Ӧ���Դ˷������
��1����������������A��������2������1��3������ϩ��B�Ľṹ��ʽ��CH2=CH-COOC2H5��
��ˣ�������ȷ���ǣ�2������1��3������ϩ��CH2=CH-COOCH2CH3��
��2��A�Ľṹ��ʽΪCH2=C(CH3)-CH=CH2��A������Ȳ����ͬ���칹����CH![]() C-CH2CH2CH3��CH3-C
C-CH2CH2CH3��CH3-C![]() C-CH2CH3��CH
C-CH2CH3��CH![]() C-CH��CH3��2��3�֣�
C-CH��CH3��2��3�֣�
������Ϣ�еķ�Ӧ������CH2=C(CH3)-CH=CH2��CH2=CH-COOC2H5�ڼ��������·�Ӧ�������� ��
��
��ˣ�������ȷ���ǣ�3�� ��
��
��3���������Ϸ�������Ӧ��Ϊ̼̼˫���ļӳɷ�Ӧ����Ӧ��Ϊ��ȥ±�������ȥ��Ӧ����ѧ����ʽΪ�� ��
��
��ˣ�������ȷ���ǣ��ӳɷ�Ӧ�� ��
��
��4��B�Ľṹ��ʽΪCH2=CH-COOC2H5��B��ͬ���칹������������������ܷ���������Ӧ˵������ȩ�������������ӽṹ�к������������������ܷ���ˮ�ⷴӦ˵�������������ۺϣ�����������������Ӧ����HCOO-�������������ͬ���칹���У�HCOO-C(CH3)=CHCH3 ��HCOO-CH=CH(CH3)2��
��ˣ�������ȷ���ǣ�HCOO-C(CH3)=CHCH3 ��HCOO-CH=CH(CH3)2��
��5�����CH2=CH-COOH�Ľṹ������1��2-���ȱ�����ˮ������C(1��2-������)��C����������X��X��һ������Y��Y���ԭ�õ�Z��Z������ȥ��Ӧ�õ�CH2=CH-COOH����������Ϊ��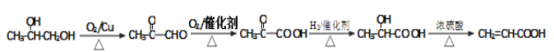 ��
��
��ˣ�������ȷ���ǣ�
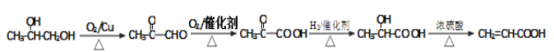 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�