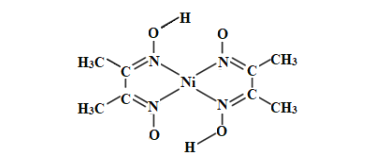
��Ŀ����
����Ŀ����7�֣�ʵ�����ü��ȹ����Ȼ�狀��������ƵĻ������ȡ������Ӧ�Ļ�ѧ����ʽΪ�� ��
���Ƶ������г����������������壬Ϊ�˵õ������İ��������Լ��飬��������װ�û��Լ��У�����Ҫ�������ʵ�ѡ���������ĸ������еĿո�
��1��װ�ã�

��2���Լ��� a��NaOH��Һ b������ʯ��ˮ c��Ũ���� d������NaHCO3��Һ
e����ʯ�� f��Ʒ����Һ g��ʪ��ĺ�ɫʯ����ֽ
��ȡ���� | �������� | ��ȡ����ķ���װ�� | ��ȥ��������ľ���װ�� | �ռ�װ�� | ����װ���� �����Լ� | �����Ƶ����������Լ� |
NH3 | H2O(g) |
���𰸡�Ca(OH)2 + 2NH4Cl ![]() CaCl2 + 2NH3��+ 2H2O��2���� �����¸���1����
CaCl2 + 2NH3��+ 2H2O��2���� �����¸���1����
NH3 | H2O��g�� | A | G | F | e | g |
��������ʵ������ȡ�����ķ���ʽΪCa(OH)2 + 2NH4Cl ![]() CaCl2 + 2NH3��+ 2H2O��
CaCl2 + 2NH3��+ 2H2O��
������ȡ������ԭ����֪������װ�����ڹ������֮�������ȡ����ģ�����ѡ��A�������Ǽ������壬����Ӧ���ü�ʯ�ң���ʯ�ҿ��Է��ڸ�����ڣ�����G��e��������������ˮ����������ˮ���ռ����������ܶ�С�ڿ����ģ��������ſ������ռ��������Ǽ������壬����ˮ�Լ��ԣ�������ʪ��ĺ�ɫʯ����ֽ���顣

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�����Ŀ���й���ѧԺ������ѧ�����о��������״���ȡ��̼ϩ��(![]() ��������ù��ҿ�ѧ��������һ�Ƚ����ü�������ú�����ƺϳ������ɺϳ����Ƽ״��Ͱ���������ɼ״�����ϩ�ͱ�ϩ��
��������ù��ҿ�ѧ��������һ�Ƚ����ü�������ú�����ƺϳ������ɺϳ����Ƽ״��Ͱ���������ɼ״�����ϩ�ͱ�ϩ��
(1)ú��������һϵ�л�ѧ��Ӧ����֪�Ȼ�ѧ����ʽ��
![]()
![]()
![]()
![]()
��Ӧ![]()
![]() _____
_____![]()
(2)��Ͷ�ϱ�![]() �ϳɼ״���
�ϳɼ״���![]() ���¶ȡ�ѹǿ��
���¶ȡ�ѹǿ��![]() ��ƽ��ת���ʵĹ�ϵ��ͼ��ʾ
��ƽ��ת���ʵĹ�ϵ��ͼ��ʾ

�ش��������⣺
��![]() _____(���������)0
_____(���������)0
��![]() ______(����������������)
______(����������������)![]()
��![]() ��
��![]() ʱ��
ʱ��![]() ���
���![]() _____(��>����<��)
_____(��>����<��)![]() ��
��
������ʼʱ���Ͷ�ϱ�![]() ��ƽ��ת���ʽ�_______(����������������С��)��
��ƽ��ת���ʽ�_______(����������������С��)��
�ݲ����±���ʵ�ʹ�ҵ�����д�����![]() ��
��![]() �����ŵ���____________��
�����ŵ���____________��
span>���� | ���� | ���� | ��ע | �ص� | |
ѹ�� | �¶� | ||||
��ѹ�� |
��Ԫ���� | 25~30 | 380~400 | 1924�깤ҵ�� | (1)���������ж����������� (2)����Ӧ�� |
��ѹ�� |
��Ԫ���� | 5 | 230~270 | 1966�깤ҵ�� | (1)�������ж����������ף�����Ϊ1~2�� (2)����Ӧ�� |
(3)![]() ��
��![]() ����
����![]() �ķ�ӦΪ
�ķ�ӦΪ![]()
![]() ����
����![]() ���������µķ�Ӧ��������(
���������µķ�Ӧ��������(![]() ��ʾ����̬)
��ʾ����̬)
��ѧ������![]() ��
��![]()
���淴Ӧ![]() ��
��![]() ��
��![]()
�Ѹ���![]()
���У�![]() �������ֽⷴӦ��ܸߣ��������������˺ϳɰ������巴Ӧ����.
�������ֽⷴӦ��ܸߣ��������������˺ϳɰ������巴Ӧ����.
����������ߺϳɰ�ƽ����ʵ�������______(����ĸ)
A ���� B ���� C ��ѹ D ��ѹ E ����
�ڱ�ƽ�ⳣ��![]() ��
��![]() ������
������![]() Ϊ��ѹǿ(
Ϊ��ѹǿ(![]() )��
)��![]() ��
��![]() ��
��![]() Ϊ����ֵ�ƽ���ѹ����
Ϊ����ֵ�ƽ���ѹ����![]() ��
��![]() Ϊƽ����ѹ��
Ϊƽ����ѹ��![]() Ϊƽ��ϵͳ��
Ϊƽ��ϵͳ��![]() �����ʵ�������.��֪��ʼʱ��һ�ܱ�������Ͷ��
�����ʵ�������.��֪��ʼʱ��һ�ܱ�������Ͷ��![]()
![]() ��
��![]()
![]() ����Ӧ
����Ӧ![]()
![]() ��
��![]()
![]()
![]()
![]() �ں㶨�¶Ⱥͱ�ѹǿ�½��У�
�ں㶨�¶Ⱥͱ�ѹǿ�½��У�![]() ��ƽ�����Ϊ
��ƽ�����Ϊ![]() ����÷�Ӧ��
����÷�Ӧ��![]() ��________(�ú�
��________(�ú�![]() �Ĵ���ʽ��ʾ)����ͼ�п��Ա�ʾ��ƽ�ⳣ�����¶�
�Ĵ���ʽ��ʾ)����ͼ�п��Ա�ʾ��ƽ�ⳣ�����¶�![]() �ı仯���Ƶ���____________(����ĸ)
�ı仯���Ƶ���____________(����ĸ)