��Ŀ����
�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ���塣
��1����CO2�뽹̿��������CO��CO�����������ȡ�
��֪��Fe2O3(s)+3C(ʯī)=2Fe(s)+3CO(g) ��H 1=+489.0 kJ��mol��1
C(ʯī)+CO2(g)=2CO(g) ��H 2=+172.5 kJ��mol��1
��CO��ԭFe2O3(s)���Ȼ�ѧ����ʽΪ ��
��2��������̼�ϳɼ״���̼���ŵ��·���CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��
CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H
CH3OH(g)+H2O(g) ��H
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK= ��
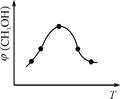
��ȡһ�����CO2��H2�Ļ������(���ʵ���֮��Ϊ1��3)����������ܱ������У�����������Ӧ����Ӧ�����в�ü״������������(CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ��ͼ��ʾ����÷�Ӧ�Ħ�H 0(�>������<������)��
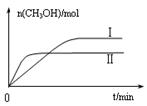
�������ֲ�ͬ�����·�����Ӧ�����CH3OH�����ʵ�����ʱ��仯��ͼ��ʾ������I�����Ӧ��ƽ�ⳣ����С��ϵΪK�� K��(�>������<������)��
��3����CO2Ϊԭ�ϻ����Ժϳɶ������ʡ�
�ٹ�ҵ������[CO(NH2)2]��CO2��NH3��һ�������ºϳɣ��䷴Ӧ����ʽΪ ������̼��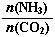 ��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ
��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ
��
����������Һ������ʽ��е�⣬CO2�ڵ缫�Ͽ�ת��Ϊ���飬�õ缫��Ӧ�ķ���ʽΪ ��
��1����CO2�뽹̿��������CO��CO�����������ȡ�
��֪��Fe2O3(s)+3C(ʯī)=2Fe(s)+3CO(g) ��H 1=+489.0 kJ��mol��1
C(ʯī)+CO2(g)=2CO(g) ��H 2=+172.5 kJ��mol��1
��CO��ԭFe2O3(s)���Ȼ�ѧ����ʽΪ ��
��2��������̼�ϳɼ״���̼���ŵ��·���CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��
CO2(g)+3H2(g)
 CH3OH(g)+H2O(g) ��H
CH3OH(g)+H2O(g) ��H�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK= ��
��ȡһ�����CO2��H2�Ļ������(���ʵ���֮��Ϊ1��3)����������ܱ������У�����������Ӧ����Ӧ�����в�ü״������������(CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ��ͼ��ʾ����÷�Ӧ�Ħ�H 0(�>������<������)��

�������ֲ�ͬ�����·�����Ӧ�����CH3OH�����ʵ�����ʱ��仯��ͼ��ʾ������I�����Ӧ��ƽ�ⳣ����С��ϵΪK�� K��(�>������<������)��

��3����CO2Ϊԭ�ϻ����Ժϳɶ������ʡ�
�ٹ�ҵ������[CO(NH2)2]��CO2��NH3��һ�������ºϳɣ��䷴Ӧ����ʽΪ ������̼��
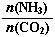 ��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ
��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ��
����������Һ������ʽ��е�⣬CO2�ڵ缫�Ͽ�ת��Ϊ���飬�õ缫��Ӧ�ķ���ʽΪ ��
��1��Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H=��28.5 kJ��mol��1��2�֣�
��2����
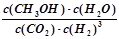 ��2�֣� �ڣ���2�֣� ��>��2�֣�
��2�֣� �ڣ���2�֣� ��>��2�֣���3����2NH3+CO2
 CO(NH2)2+H2O��2�֣� 40%��2�֣�
CO(NH2)2+H2O��2�֣� 40%��2�֣���CO2+8e��+8H+=CH4+2H2O��2�֣�
�����������1����Ӧ�٣� �ڡ�3�ɵ�Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H=��28.5 kJ��mol��1����2���ڸ�ͼ���Ƿ�Ӧ����ͼ����ߵ�ǰ��δƽ��ʱ�ı仯����ߵ�����ƽ��仯�����¶�����ƽ��������У���Ӧ���ȣ�����ͼ�ж����ߢ��Ӧ�ķ�Ӧ��Ӧ���ʿ죬�¶ȸߣ��״������ͣ�KֵС����3���ٷ�ӦΪ2NH3+CO2
 CO(NH2)2+H2O���谱�������ʵ���Ϊ3mol���������̼����Ϊ1mol����
CO(NH2)2+H2O���谱�������ʵ���Ϊ3mol���������̼����Ϊ1mol����2NH3+CO2
 CO(NH2)2+H2O
CO(NH2)2+H2Oʼ�� 3 1 0 0
ת���� 1.2 0.6 0.6 0.6
ƽ���� 1.8 0.4 0.6 0.6
��NH3��ƽ��ת����Ϊ1.2��3=0.4
�ڸ�����Ϣ�������Խ����з�ӦΪCO2+8e��+8H+=CH4+2H2O��

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
 2H2O
2H2O  CH3OH(g) ��H1����90.7 kJ��mol��1����
CH3OH(g) ��H1����90.7 kJ��mol��1����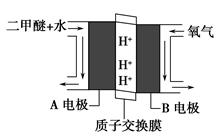
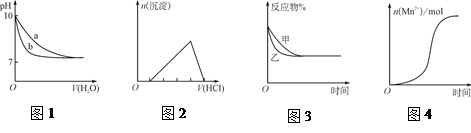
 CO2(g)��H2(g)��Ӱ�죬�ҵ�ѹǿ�ȼ�ѹǿ��
CO2(g)��H2(g)��Ӱ�죬�ҵ�ѹǿ�ȼ�ѹǿ�� 4NO2(g)+O2(g)����H��0����Ӧ��5����ʱ�����ʵ�Ũ�Ȳ��ٷ����仯�����NO2���������Ϊ50%��
4NO2(g)+O2(g)����H��0����Ӧ��5����ʱ�����ʵ�Ũ�Ȳ��ٷ����仯�����NO2���������Ϊ50%�� H2NCOONH4(���������)(l) ��H1
H2NCOONH4(���������)(l) ��H1
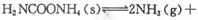
 ��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
��ʵ���ò�ͬ�¶��µ�ƽ�����������±���