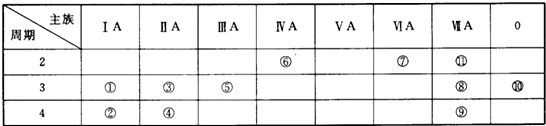
��Ŀ����
9��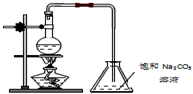 ��ͼΪ��ȡ����������ʵ��װ��ͼ����ش��������⣺
��ͼΪ��ȡ����������ʵ��װ��ͼ����ش��������⣺��1��ʵ������ȡ���������Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��2������������ת���ʣ��ɲ�ȡ�Ĵ�ʩ���Ҵ���������ʱ��������������ȣ�
��3������ͼ��ʾ��װ������ȡ������������������������ƫ�ͣ���ԭ������ǣ�ԭ����������Ӧ�ͱ��������¶ȹ��ߣ������˸���Ӧ������Ч�����ã����ֲ���ӷ��˵ȣ�
��4��ʵ��ʱ�ɹ۲쵽��ƿ�������ݲ����������ӷ���ʽ��ʾ�������ݵ�ԭ��
2CH3COOH+CO32-��2CH3COO-+CO2��+H2O��
��5���˷�Ӧ��Ũ������Ϊ���������ܻ���ɲ����������Է�Һ�������ظ�ʹ�����ѵ����⣮�ִ��о���������������Һ������˷�Ӧ�Ĵ�����ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ���
| ͬһ��Ӧʱ�� | ͬһ��Ӧ�¶� | ||||
| ��Ӧ�¶�/�� | ת���ʣ�%�� | ѡ���ԣ�%�� | ��Ӧʱ��/h | ת���ʣ�%�� | ѡ���ԣ�%�� |
| 40 | 77.8 | 100 | 2 | 80.2 | 100 |
| 60 | 92.3 | 100 | 3 | 87.7 | 100 |
| 80 | 92.6 | 100 | 4 | 92.3 | 100 |
| 120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
���ݱ������ݣ�����C�����ţ���Ϊ�÷�Ӧ�����������
A��120�棬4h B��80�棬2h C��60�棬4h D��40�棬3h��
���� ��1��ʵ������ȡ���������ķ�Ӧ��������Ӧ����Ӧ�ı���Ϊ�����ǻ��������⣬�÷�Ӧ��������������ˮ����Ϊ���淴Ӧ��
��2���÷�Ӧ��������������ˮ����Ϊ���淴Ӧ������������ת���ʣ�Ӧ�ı�����ʹƽ��������з��������ַ�Ӧ������һ�����������һ�ֵ�ת���ʣ�����������Ũ�ȴٽ�ƽ��������У�
��3�����ʺܵ͵�ԭ���У�������Ҵ����ӷ����¶ȹ���Ũ�����ܹ����Ҵ�������Ӧ�������ѻ�����ϩ��
��4����ƿ�еı���̼������Һ�����ݲ�����̼���ƺͻӷ��������ᷴӦ���ɵĶ�����̼���壻
��5�����ݱ������ݷ����¶�ת���ʵ�Ӱ�죬ѡ����ѵ��¶Ⱥͷ�Ӧʱ�䣬60��ʱ��Ӧ��ת�����Ѿ��ϸߣ���ѡ����Ϊ100%��
��� �⣺��1��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��2��CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��Ӧ�ǿ��淴Ӧ������������ת���ʿ��������Ҵ�������ƽ��������У�����ת��������Ҳ���Է���������������ٽ�ƽ��������У����������ת���ʵȣ�
�ʴ�Ϊ���Ҵ���������ʱ��������������ȣ�
��3����ͼʾ����ʵ��������Ϊԭ����������Ӧ�ͱ������������¶ȹ��ߣ������˸���Ӧ����������Ч�����ã����ֲ���ӷ��ˣ������²���ƫ�ͣ�
�ʴ�Ϊ��ԭ����������Ӧ�ͱ��������¶ȹ��ߣ������˸���Ӧ������Ч�����ã����ֲ���ӷ��˵ȣ�
��4��ʵ��ʱ�ɹ۲쵽��ƿ�������ݲ�������̼���ƺͻӷ��������ᷴӦ���ɵĶ�����̼���壬�����ӷ���ʽ��ʾ�������ݵ�ԭ��Ϊ��2CH3COOH+CO32-��2CH3COO-+CO2��+H2O��
�ʴ�Ϊ��2CH3COOH+CO32-��2CH3COO-+CO2��+H2O��
��5���ɱ����е�ͬһ��Ӧʱ��������60��ʱ��Ӧ��ת�����Ѿ��ϸߣ���ѡ����Ϊ100%��ͬһ�¶�ʱ��Ӧʱ��ѡ��4Сʱת���ʽϸߣ�ѡC��
�ʴ�Ϊ��C��
���� ���⿼�������������Ʊ�����Ŀ�Ѷ��еȣ�ע����������������Ӧԭ����ʵ����Ӧ��ע�����⣬�������ʷ��볣�÷�����������������ʵ�鷽����ƣ���������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

��1��д���ϳɼ״����Ȼ�ѧ����ʽCO��g��+2H2��g��=CH3OH��g����H=-��b-a��KJ/mol��
��2��ʵ������1L���ܱ������н���ģ��ϳ�ʵ�飮��1mol CO��2mol H2ͨ�������У��ֱ������300���500�淴Ӧ��ÿ��һ��ʱ����������CH3OH��Ũ�����±���ʾ��
| ʱ��Ũ�ȣ�mol/L���¶� | 10min | 20min | 30min | 40min | 50min | 60min |
| 300�� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500�� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
��3����500��ﵽƽ��ʱ��ƽ�ⳣ��K=25��
��4������һ���������ܱ������У�����1.2mol CO��2.0mol H2��һ�������´ﵽƽ�⣬���������ѹǿΪ��ʼѹǿ��һ�룮�����������H2��ת����Ϊ80%��
��5��ͭ���������л��Ըߡ�ѡ���Ժú������º͵��ص㣬�ѹ㷺��ʹ����CO/CO2�ļ���ϳɼ״���ʹ��ͭ�������÷�Ӧ��a�Ĵ�С�仯�Է�Ӧ�ȡ�H����Ӱ�죬��Ӱ�죮�����Ӱ�족����Ӱ�족��
��6��2009�꣬����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ����ϻ����ͻ�ƣ�ԭ����ͼb��ʾ����д����C��ͨ��O2�����ĵ缫��ӦʽO2+4e-+4H+=2H2O��
��7���������������Դ����ͼcװ�õ�ⱥ��ʳ��ˮ��C1��C2��Ϊʯī�缫����
�ٸ÷�Ӧ�����ӷ���ʽ2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2����
�ڵ�ʼ���ڵ缫C2����Χ���C1����C2�����ȳ��ֺ�ɫ��
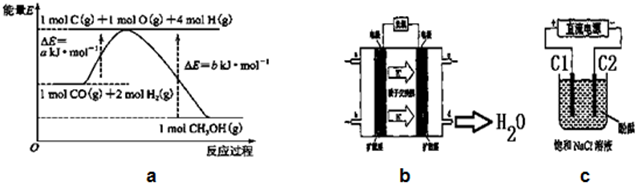
 ����ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɻ���ϩ�ķ�Ӧ
����ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɻ���ϩ�ķ�Ӧ ��ʵ��װ����ͼ��
��ʵ��װ����ͼ�������õ����й��������£�
| ��Է������� | �ܶ�/��g?cm-3 �� | �е�/�� | �ܽ��� | |
| ������ | 100 | 0.9618 | 161 | ����ˮ |
| ����ϩ | 82 | 0.8102 | 83 | ������ˮ |
��a�м���20g��������2СƬ���Ƭ����ȴ��������������1mLŨ���ᣬb��ͨ����ȴˮ��ʼ��������a�������������¶Ȳ�����90�森
�����ᴿ��
��Ӧ�ֲ��ﵹ���Һ©���зֱ�������5%̼������Һ��ˮϴ�ӣ�����������ˮ�Ȼ��ƿ���������һ��ʱ�����ȥ�Ȼ��ƣ�����ͨ������õ���������ϩ11.48g��
�ش��������⣺
��1��װ��a��������������ƿ��
��2���������Ƭ�������Ƿ�ֹ���У��������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������B������ȷ�𰸱�ţ���A����������B����ȴ��C�����貹�� D����������
��3����ʵ���������ײ����ĸ�����Ľṹ��ʽΪ
 ��
����4����Һ©����ʹ��ǰ��©������ϴ�ɾ����ڱ�ʵ���������У�����Ӧ�ôӷ�Һ©�����Ͽڵ�������Ͽڵ��������¿ڷų�������
��5�������ᴿ�����м�����ˮ�Ȼ��Ƶ�Ŀ���Ǹ��
��6���ڻ���ϩ�ֲ�����������У��������õ���������ACD������ȷ�𰸱�ţ���
A��Բ����ƿ B���¶ȼ� C������ƿ D������������ E��������
��7����ʵ�����õ��Ļ���ϩ������70%��
| A�� | �ڱ�״���£�1 molˮ�����ԼΪ22.4L | |
| B�� | ֻ���ڱ�״���£������Ħ���������22.4mol/L | |
| C�� | �ڱ�״���£�1molH2��O2�Ļ���������ԼΪ22.4 L | |
| D�� | �κ������£������Ħ���������22.4L/mol |
 ��1���������¡��˹��̵������о��������ڳ��³�ѹ����������N2�ڴ���������ˮ������Ӧ��
��1���������¡��˹��̵������о��������ڳ��³�ѹ����������N2�ڴ���������ˮ������Ӧ��