��Ŀ����
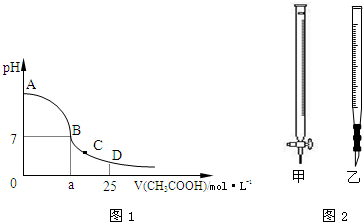
��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ҺpH�仯������ͼ1��ʾ��
��1��B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��
��2�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�ѡ�õζ�����ͼ2��ʾ��
��3����D��ʱ����Һ��c��CH3COO-��+c��CH3COOH��
��4����C�㣬��Һ������Ũ���ɴ�С��˳��Ϊ��
��1��B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��
��
��
����ǡ������������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB
AB
���AB������BC����CD���������ڣ���2�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�ѡ�õζ�����ͼ2��ʾ��
C
C
��| ��ƿ�е���Һ | �ζ����е���Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ʯ�� | �� |
| B | �� | �� | ���� | �� |
| C | �� | �� | ��̪ | �� |
| D | �� | �� | ��̪ | �� |
=
=
2c��Na+�������������������=��������4����C�㣬��Һ������Ũ���ɴ�С��˳��Ϊ��
c��CH3COO-����c��Na+����c��H+����c��OH-��
c��CH3COO-����c��Na+����c��H+����c��OH-��
��
��������1��������ǡ����ȫ��Ӧ�������˴����ƣ���ҺӦ����ʾ���Է�����
��2��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ƿ��ʢ������������Һ���ζ�����ʢ�Ŵ�����Һ��ָʾ��Ӧ��ѡ�÷�̪��
��3�����������غ���з�����
��4��C������������Һ��ʾ���ԣ����ݵ���غ��ж���Һ�и�����Ũ�ȴ�С��
��2��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ƿ��ʢ������������Һ���ζ�����ʢ�Ŵ�����Һ��ָʾ��Ӧ��ѡ�÷�̪��
��3�����������غ���з�����
��4��C������������Һ��ʾ���ԣ����ݵ���غ��ж���Һ�и�����Ũ�ȴ�С��
����⣺��1��NaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧʱ�������˴����ƣ����������ˮ�⣬��Һ��ʾ���ԣ���Һ��pH����7������ǡ����ȫ��Ӧʱ��Һ��pH����7������Ӧ����AB�Σ�
�ʴ�Ϊ����AB��
��2�����������Ϣ����ƿ��Ӧ��ʢ������������Һ���ζ�����ʢ�Ŵ��ᣬ����ǡ�÷�Ӧʱ��Һ��ʾ���ԣ�Ӧ��ʹ�÷�̪��Ϊָʾ��������C��ȷ��
��ѡC��
��3��D��ʱ��������Ϊ25mL��n��CH3COO-��+n��CH3COOH��=0.2mol?L-1��0.025L=0.005mol��
n��Na+��=0.1mol?L-1��0.025L=0.0025mol������n��CH3COO-��+n��CH3COOH��=2n��Na+����
������Һ�������ͬ������c��CH3COO-��+c��CH3COOH��=2c��Na+����
�ʴ�Ϊ��=��
��4����C�㣬��Һ��pH��7��������Ũ�ȴ�������������Ũ�ȣ����ݵ���غ㣺CH3COO-+OH-=Na++H+��֪����Һ�У�c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ����AB��
��2�����������Ϣ����ƿ��Ӧ��ʢ������������Һ���ζ�����ʢ�Ŵ��ᣬ����ǡ�÷�Ӧʱ��Һ��ʾ���ԣ�Ӧ��ʹ�÷�̪��Ϊָʾ��������C��ȷ��
��ѡC��
��3��D��ʱ��������Ϊ25mL��n��CH3COO-��+n��CH3COOH��=0.2mol?L-1��0.025L=0.005mol��
n��Na+��=0.1mol?L-1��0.025L=0.0025mol������n��CH3COO-��+n��CH3COOH��=2n��Na+����
������Һ�������ͬ������c��CH3COO-��+c��CH3COOH��=2c��Na+����
�ʴ�Ϊ��=��
��4����C�㣬��Һ��pH��7��������Ũ�ȴ�������������Ũ�ȣ����ݵ���غ㣺CH3COO-+OH-=Na++H+��֪����Һ�У�c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
���������⿼�����к͵ζ�����Ŀ�ѶȲ���ע�������к͵ζ�������ָʾ����ѡ�÷������Ƚ�����Ũ�ȴ�Сʱ��ע�����غ�������غ��Ӧ�ã�������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д�
�����Ŀ
 ��25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L���ᣬ��������ͼ��ʾ���й�����������ǣ�������
��25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L���ᣬ��������ͼ��ʾ���й�����������ǣ�������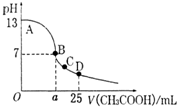 ��2011?ӥ̶��ģ�������£���25mL 0.1mol��L-1NaOH��Һ����μ���0.2mol?L-1 CH3COOH ��Һ��pH ��μ� CH3COOH��Һ����Ĺ�ϵ������ͼ��ʾ������������Һ���ʱ������仯�������й�����Ũ�ȹ�ϵ��˵��������ǣ�������
��2011?ӥ̶��ģ�������£���25mL 0.1mol��L-1NaOH��Һ����μ���0.2mol?L-1 CH3COOH ��Һ��pH ��μ� CH3COOH��Һ����Ĺ�ϵ������ͼ��ʾ������������Һ���ʱ������仯�������й�����Ũ�ȹ�ϵ��˵��������ǣ�������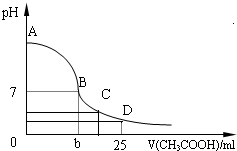 ��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ҺpH�仯������ͼ��ʾ��
��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ҺpH�仯������ͼ��ʾ��