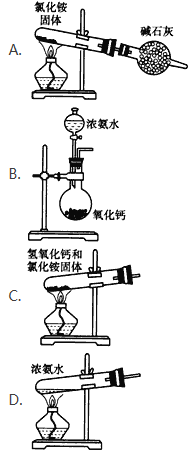
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœ¬ΆΦ «‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ§’κΕ‘±μ÷–ΒΡΔΌΓΣΔύΑΥ÷÷‘ΣΥΊΘ§”Ο‘ΣΥΊΖϊΚ≈ΜρΜ·―ß ΫΜΊ¥πœύΙΊΈ ΧβΘΚ

(l)‘Ύ’β–©‘ΣΥΊ÷–Θ§Μ·―ß–‘÷ Ήν≤ΜΜνΤΟ‘≠Ή”ΒΡ‘≠Ή”ΫαΙΙ Ψ“βΆΦΈΣ______.
(2)”ΟΒγΉ” Ϋ±μ ΨΔΌ”κΔέ–Έ≥…Μ·ΚœΈοΒΡΙΐ≥Χ______.ΘΚ
(3)’β–©‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο÷–Θ§Υα–‘Ήν«ΩΒΡ «______. Θ§ΔΎΓΔΔήΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΒΡ»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ______.
(4)ΔΌΔΎΔέΔήΥΡ÷÷‘ΣΥΊΒΡΦρΒΞάκΉ”ΑκΨΕ”…¥σΒΫ–ΓΒΡΥ≥–ρ «______.Θ®”ΟάκΉ”ΖϊΚ≈ΚΆΓΑ>Γ±±μ ΨΘ©ΓΘ
(5)ΔΌΔίΔύ»ΐ÷÷‘ΣΥΊΒΡΒΞ÷ Ζ–Βψ”…ΗΏΒΫΒΆΒΡΥ≥–ρ «______.Θ®”ΟΜ·―ß ΫΚΆΓΑ>Γ±±μ ΨΘ©ΓΘ
ΓΨ¥πΑΗΓΩ 
![]() HClO4 Al(OH)3ΘΪOHΘ≠ΘΫAlO2Θ≠ΘΪ2H2O FΘ≠ΘΨNaΘΪΘΨMg2ΘΪΘΨAl3ΘΪ Br2ΘΨCl2ΘΨF2
HClO4 Al(OH)3ΘΪOHΘ≠ΘΫAlO2Θ≠ΘΪ2H2O FΘ≠ΘΨNaΘΪΘΨMg2ΘΪΘΨAl3ΘΪ Br2ΘΨCl2ΘΨF2
ΓΨΫβΈωΓΩΗυΨί‘ΣΥΊ‘Ύ÷ήΤΎ±μ÷–ΒΡœύΕ‘ΈΜ÷ΟΩ…≈–ΕœΔΌΓΪΔύΑΥ÷÷‘ΣΥΊΖ÷±π «FΓΔNaΓΔMgΓΔAlΓΔClΓΔArΓΔKΓΔBrΓΘ‘ρ
Θ®1Θ©‘Ύ’β–©‘ΣΥΊ÷–Θ§Μ·―ß–‘÷ Ήν≤ΜΜνΤΟ‘≠Ή” «œΓ”–ΤχΧεArΘ§‘≠Ή”ΫαΙΙ Ψ“βΆΦΈΣ ΘΜΘ®2Θ©ΔΌ”κΔέ–Έ≥…ΒΡΜ·ΚœΈο «ΖζΜ·ΟΨΘ§Κ§”–άκΉ”ΦϋΒΡάκΉ”Μ·ΚœΈοΘ§–Έ≥…Ιΐ≥Χ «
ΘΜΘ®2Θ©ΔΌ”κΔέ–Έ≥…ΒΡΜ·ΚœΈο «ΖζΜ·ΟΨΘ§Κ§”–άκΉ”ΦϋΒΡάκΉ”Μ·ΚœΈοΘ§–Έ≥…Ιΐ≥Χ «![]() ΘΜΘ®3Θ©Ζ«Ϋπ τ–‘‘Ϋ«ΩΘ§ΉνΗΏΦέΚ§―θΥαΒΡΥα–‘‘Ϋ«ΩΘ§FΟΜ”–Κ§―θΥαΘ§‘ρ’β–©‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο÷–Θ§Υα–‘Ήν«ΩΒΡ «HClO4ΘΜΔΎΓΔΔήΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΖ÷±π ««β―θΜ·ΡΤΚΆ«β―θΜ·¬ΝΘ§Εΰ’ΏΒΡ»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣAl(OH)3ΘΪOHΘ≠ΘΫAlO2Θ≠ΘΪ2H2OΘΜΘ®4Θ©ΔΌΔΎΔέΔήΥΡ÷÷‘ΣΥΊΒΡΦρΒΞάκΉ”ΚΥΆβΒγΉ”≈≈≤ΦœύΆ§Θ§άκΉ”ΑκΨΕΥφ‘≠Ή”–ρ ΐΒΡ‘ω¥σΕχΦθ–ΓΘ§‘ράκΉ”ΑκΨΕ”…¥σΒΫ–ΓΒΡΥ≥–ρ «FΘ≠ΘΨNaΘΪΘΨMg2ΘΪΘΨAl3ΘΪΓΘΘ®5Θ©¬±ΥΊΒΞ÷ Ή‘…œΕχœ¬»έΖ–Βψ÷πΫΞ…ΐΗΏΘ§‘ρΔΌΔίΔύ»ΐ÷÷‘ΣΥΊΒΡΒΞ÷ Ζ–Βψ”…ΗΏΒΫΒΆΒΡΥ≥–ρ «Br2ΘΨCl2ΘΨF2ΓΘ
ΘΜΘ®3Θ©Ζ«Ϋπ τ–‘‘Ϋ«ΩΘ§ΉνΗΏΦέΚ§―θΥαΒΡΥα–‘‘Ϋ«ΩΘ§FΟΜ”–Κ§―θΥαΘ§‘ρ’β–©‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο÷–Θ§Υα–‘Ήν«ΩΒΡ «HClO4ΘΜΔΎΓΔΔήΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΖ÷±π ««β―θΜ·ΡΤΚΆ«β―θΜ·¬ΝΘ§Εΰ’ΏΒΡ»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣAl(OH)3ΘΪOHΘ≠ΘΫAlO2Θ≠ΘΪ2H2OΘΜΘ®4Θ©ΔΌΔΎΔέΔήΥΡ÷÷‘ΣΥΊΒΡΦρΒΞάκΉ”ΚΥΆβΒγΉ”≈≈≤ΦœύΆ§Θ§άκΉ”ΑκΨΕΥφ‘≠Ή”–ρ ΐΒΡ‘ω¥σΕχΦθ–ΓΘ§‘ράκΉ”ΑκΨΕ”…¥σΒΫ–ΓΒΡΥ≥–ρ «FΘ≠ΘΨNaΘΪΘΨMg2ΘΪΘΨAl3ΘΪΓΘΘ®5Θ©¬±ΥΊΒΞ÷ Ή‘…œΕχœ¬»έΖ–Βψ÷πΫΞ…ΐΗΏΘ§‘ρΔΌΔίΔύ»ΐ÷÷‘ΣΥΊΒΡΒΞ÷ Ζ–Βψ”…ΗΏΒΫΒΆΒΡΥ≥–ρ «Br2ΘΨCl2ΘΨF2ΓΘ

 ΑΌΡξ―ßΒδΩΈ ±―ßΝΖ≤βœΒΝ–¥πΑΗ
ΑΌΡξ―ßΒδΩΈ ±―ßΝΖ≤βœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΓΨ2017ΫλΫ≠Υ’ ΓΚΘΑ≤ΗΏΦΕ÷–―ßΗΏ»ΐ‘¬ΩΦΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§‘Ύ»ΐΗω»ίΜΐΨυΈΣ2.0 LΒΡΚψ»ίΟή±’»ίΤς÷–ΖΔ…ζΖ¥”ΠΘΚ
2NO(g)ΘΪ2CO(g)![]() N2(g)ΘΪ2CO2(g)
N2(g)ΘΪ2CO2(g)
Ης»ίΤς÷–Τπ ΦΈο÷ ΒΡΝΩ”κΖ¥”ΠΈ¬Ε»»γœ¬±μΥυ ΨΘ§Ζ¥”ΠΙΐ≥Χ÷–ΦΉΓΔ±ϊ»ίΤς÷–CO2ΒΡΈο÷ ΒΡΝΩΥφ ±Φδ±δΜ·ΙΊœΒ»γΆΦΥυ ΨΘΚ
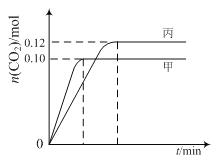
»ίΤς | Έ¬Ε»/Γφ | Τπ ΦΈο÷ ΒΡΝΩ/mol | |
NO (g) | CO (g) | ||
ΦΉ | T1 | 0.20 | 0.20 |
““ | T1 | 0.30 | 0.30 |
±ϊ | T2 | 0.20 | 0.20 |
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
AΘ°ΗΟΖ¥”ΠΒΡ’ΐΖ¥”ΠΈΣΈϋ»»Ζ¥”Π
BΘ°¥οΒΫΤΫΚβ ±Θ§““÷–CO2ΒΡΧεΜΐΖ÷ ΐ±»ΦΉ÷–ΒΡ–Γ
CΘ°T1Γφ ±Θ§»τΤπ Φ ±œρΦΉ÷–≥δ»κ0.40 mol NOΓΔ0.40mol COΓΔ0.40mol N2ΚΆ0.40mol CO2Θ§‘ρΖ¥”Π¥οΒΫ–¬ΤΫΚβ«Αv(’ΐ)ΘΨv(Ρφ)
DΘ°T2Γφ ±Θ§»τΤπ Φ ±œρ±ϊ÷–≥δ»κ0.06mol N2ΚΆ0.12 mol CO2Θ§‘ρ¥οΤΫΚβ ±N2ΒΡΉΣΜ·¬ ¥σ”Ύ40%
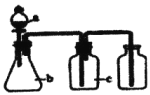
ΓΨΧβΡΩΓΩœ¬±μ÷–aΓΔbΓΔc±μ Ψœύ”Π“«Τς÷–Φ”»κΒΡ ‘ΦΝΘ§Ω…”Ο»γΆΦΉΑ÷Ο÷Τ»ΓΓΔΨΜΜ·ΓΔ ’Φ·ΒΡΤχΧε «:
―Γœν | ΤχΧε | a | b | c |
|
A | NH3 | ≈®Α±Υ° | …ζ ·Μ“ | Φν ·Μ“ | |
B | CO2 | ―ΈΥα | ΧΦΥαΗΤ | ±ΞΚΆNaHCO3»ή“Κ | |
C | NO | œΓœθΥα | Ά≠–Φ | H2O | |
D | Cl2 | ≈®―ΈΥα | Εΰ―θΜ·ΟΧ | ±ΞΚΆNaCl»ή“Κ |
A. A B. B C. C D. D