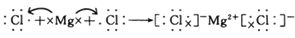��Ŀ����
��9�֣���A��B��C��D��E����Ԫ�أ�AԪ���γɵģ�2�������ӱȺ��ĺ����������8����BԪ�ص�һ��������Ϊ����ɫ���壬�ù�����������������A�ĵ��ʡ�CΪԭ�Ӻ�����12�����ӵĶ��۽�������2.4 g C���������ᷴӦʱ�����ɱ�״���µ�H2 2.24 L��D��ԭ��M������7�����ӣ�E��Aͬ�����������ȴ�����3�����ӡ�
��1��C���ӵĽṹʾ��ͼ ��
��2��A��E�⻯����ȶ��ԱȽ� �����ѧʽ��
��3��A��B��C��D�������Ӱ뾶�ɴ�С˳�� �������ӷ��ű�ʾ����
��4���õ���ʽ��ʾC��D�γɻ�������γɹ��̣�
��
��5��д��D������B������������Ӧˮ���ﷴӦ�����ӷ���ʽ��
��
��6��д��Cu��E������������Ӧˮ����ϡ��Һ��Ӧ�����ӷ���ʽ��
��
��1��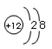 ��1�֣� ��2��H2O>NH3��1�֣�
��1�֣� ��2��H2O>NH3��1�֣�
��3��Cl->O2->Na+>Mg2+��1�֣�
��4����2�֣�

��5��Cl2+2OH-=Cl-+ClO-+H2O��2�֣� ��6��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��2�֣�
��������AԪ���γɵģ�2�������ӱȺ��ĺ����������8������A����Ԫ�ء�BԪ�ص�һ��������Ϊ����ɫ���壬�ù�����������������A�ĵ��ʣ����B����Ԫ�ء�C�����ԭ��������24��������������12������þԪ�ء�D��ԭ��M������7�����ӣ�����D����Ԫ�ء�E��Aͬ�����������ȴ�����3�����ӣ���Ԫ�ء�
��1��þ���Ӻ��������10�����ṹʾ��ͼΪ ��
��
��2���ǽ�����Խǿ����Ӧ�⻯����ȶ���Խǿ����Ԫ�صķǽ�����ǿ�ڵ�Ԫ�صģ�����ˮ���ȶ���ǿ�ڰ����ġ�
��3����������Ų���ͬ�����������뾶��ԭ���������������С������˳��Ϊ
Cl->O2->Na+>Mg2+��
��4���Ȼ�þ�������Ӽ��γɵ����ӻ�������γɹ��̿ɱ�ʾΪ